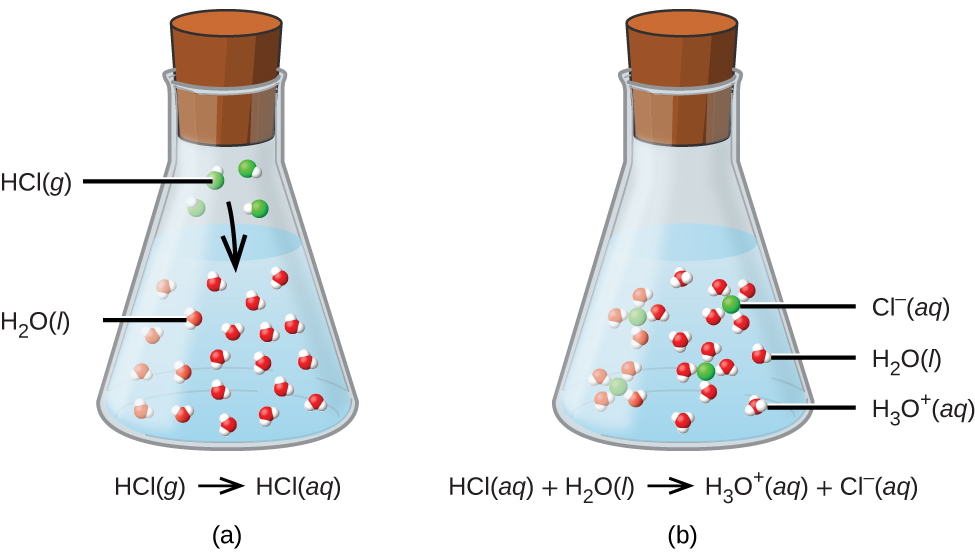
Do Bases React With All Metals . some metal reacts with a base to form salts and hydrogen (h 2) gas. Essentially, acids accept electron pairs and donate hydrogen protons. Most metals do not react with bases but zinc(zn) metal does. bases do not react with metals in the way that acids do. bases also react with certain metals, like zinc or aluminum, to produce hydrogen gas. What are acids and bases? Complexes of metal ions with water, ammonia, and hydroxide ion are examples. There are currently three definitions for acids and bases that entail how they behave when placed in solutions. So what is an acid? all metal cations are potential lewis acids. Bases react with acids to produce a salt and water. They release energy to the surroundings and the released energy is associated with the.
from wisc.pb.unizin.org
Bases react with acids to produce a salt and water. So what is an acid? There are currently three definitions for acids and bases that entail how they behave when placed in solutions. all metal cations are potential lewis acids. Essentially, acids accept electron pairs and donate hydrogen protons. They release energy to the surroundings and the released energy is associated with the. bases also react with certain metals, like zinc or aluminum, to produce hydrogen gas. Complexes of metal ions with water, ammonia, and hydroxide ion are examples. bases do not react with metals in the way that acids do. Most metals do not react with bases but zinc(zn) metal does.
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW
Do Bases React With All Metals What are acids and bases? They release energy to the surroundings and the released energy is associated with the. some metal reacts with a base to form salts and hydrogen (h 2) gas. So what is an acid? Complexes of metal ions with water, ammonia, and hydroxide ion are examples. Bases react with acids to produce a salt and water. What are acids and bases? all metal cations are potential lewis acids. bases also react with certain metals, like zinc or aluminum, to produce hydrogen gas. Most metals do not react with bases but zinc(zn) metal does. bases do not react with metals in the way that acids do. There are currently three definitions for acids and bases that entail how they behave when placed in solutions. Essentially, acids accept electron pairs and donate hydrogen protons.
From www.vedantu.com
Reactions with Metals Important Concepts and Tips for JEE Do Bases React With All Metals some metal reacts with a base to form salts and hydrogen (h 2) gas. So what is an acid? They release energy to the surroundings and the released energy is associated with the. all metal cations are potential lewis acids. Essentially, acids accept electron pairs and donate hydrogen protons. bases do not react with metals in the. Do Bases React With All Metals.
From www.masterorganicchemistry.com
Introduction to AcidBase Reactions Master Organic Chemistry Do Bases React With All Metals There are currently three definitions for acids and bases that entail how they behave when placed in solutions. bases also react with certain metals, like zinc or aluminum, to produce hydrogen gas. bases do not react with metals in the way that acids do. Bases react with acids to produce a salt and water. Complexes of metal ions. Do Bases React With All Metals.
From www.youtube.com
How do bases react with metals? Reaction of base and metal YouTube Do Bases React With All Metals bases do not react with metals in the way that acids do. There are currently three definitions for acids and bases that entail how they behave when placed in solutions. What are acids and bases? some metal reacts with a base to form salts and hydrogen (h 2) gas. Complexes of metal ions with water, ammonia, and hydroxide. Do Bases React With All Metals.
From www.sliderbase.com
AcidBase Reactions Presentation Chemistry Do Bases React With All Metals What are acids and bases? There are currently three definitions for acids and bases that entail how they behave when placed in solutions. Essentially, acids accept electron pairs and donate hydrogen protons. all metal cations are potential lewis acids. some metal reacts with a base to form salts and hydrogen (h 2) gas. bases do not react. Do Bases React With All Metals.
From www.chemistrystudent.com
Group 2 Metal Reactions (ALevel) ChemistryStudent Do Bases React With All Metals bases also react with certain metals, like zinc or aluminum, to produce hydrogen gas. They release energy to the surroundings and the released energy is associated with the. There are currently three definitions for acids and bases that entail how they behave when placed in solutions. some metal reacts with a base to form salts and hydrogen (h. Do Bases React With All Metals.
From hamdanano.blogspot.com
Metal And Acid Reaction How acids react with metal carbonates CBSE Do Bases React With All Metals What are acids and bases? They release energy to the surroundings and the released energy is associated with the. Most metals do not react with bases but zinc(zn) metal does. There are currently three definitions for acids and bases that entail how they behave when placed in solutions. Bases react with acids to produce a salt and water. So what. Do Bases React With All Metals.
From www.numerade.com
Metal oxides can react with water to form bases. Do Bases React With All Metals Complexes of metal ions with water, ammonia, and hydroxide ion are examples. Bases react with acids to produce a salt and water. all metal cations are potential lewis acids. bases do not react with metals in the way that acids do. So what is an acid? What are acids and bases? some metal reacts with a base. Do Bases React With All Metals.
From www.youtube.com
How do acids and bases react with metals class 10 Chapter 2 Acids Do Bases React With All Metals Complexes of metal ions with water, ammonia, and hydroxide ion are examples. all metal cations are potential lewis acids. Most metals do not react with bases but zinc(zn) metal does. They release energy to the surroundings and the released energy is associated with the. What are acids and bases? bases do not react with metals in the way. Do Bases React With All Metals.
From mavink.com
Acid Base Chemical Reaction Examples Do Bases React With All Metals bases also react with certain metals, like zinc or aluminum, to produce hydrogen gas. bases do not react with metals in the way that acids do. all metal cations are potential lewis acids. They release energy to the surroundings and the released energy is associated with the. Complexes of metal ions with water, ammonia, and hydroxide ion. Do Bases React With All Metals.
From www.yaclass.in
How do bases react with metals — lesson. Science CBSE, Class 10. Do Bases React With All Metals all metal cations are potential lewis acids. bases also react with certain metals, like zinc or aluminum, to produce hydrogen gas. bases do not react with metals in the way that acids do. They release energy to the surroundings and the released energy is associated with the. Most metals do not react with bases but zinc(zn) metal. Do Bases React With All Metals.
From readthescience.blogspot.com
READ THE SCIENCE 10.1 The reaction of metals with oxygen Do Bases React With All Metals some metal reacts with a base to form salts and hydrogen (h 2) gas. So what is an acid? There are currently three definitions for acids and bases that entail how they behave when placed in solutions. all metal cations are potential lewis acids. Complexes of metal ions with water, ammonia, and hydroxide ion are examples. Most metals. Do Bases React With All Metals.
From www.slideserve.com
PPT METAL REACTIONS PowerPoint Presentation, free download ID2670563 Do Bases React With All Metals bases do not react with metals in the way that acids do. They release energy to the surroundings and the released energy is associated with the. bases also react with certain metals, like zinc or aluminum, to produce hydrogen gas. Most metals do not react with bases but zinc(zn) metal does. What are acids and bases? Bases react. Do Bases React With All Metals.
From www.teachoo.com
How do Acids and Bases react with Metals? [with Examples] Teachoo Do Bases React With All Metals Complexes of metal ions with water, ammonia, and hydroxide ion are examples. So what is an acid? bases also react with certain metals, like zinc or aluminum, to produce hydrogen gas. Bases react with acids to produce a salt and water. some metal reacts with a base to form salts and hydrogen (h 2) gas. all metal. Do Bases React With All Metals.
From www.youtube.com
How bases react with metals CBSE Class 10 Chemistry Notes YouTube Do Bases React With All Metals Complexes of metal ions with water, ammonia, and hydroxide ion are examples. bases also react with certain metals, like zinc or aluminum, to produce hydrogen gas. Essentially, acids accept electron pairs and donate hydrogen protons. all metal cations are potential lewis acids. Most metals do not react with bases but zinc(zn) metal does. They release energy to the. Do Bases React With All Metals.
From selftution.com
Reactivity Series of Metals and Nonmetals » Selftution Do Bases React With All Metals bases also react with certain metals, like zinc or aluminum, to produce hydrogen gas. bases do not react with metals in the way that acids do. They release energy to the surroundings and the released energy is associated with the. Most metals do not react with bases but zinc(zn) metal does. all metal cations are potential lewis. Do Bases React With All Metals.
From www.teachoo.com
Reaction of Metals and Nonmetals With Base Teachoo Concepts Do Bases React With All Metals So what is an acid? Essentially, acids accept electron pairs and donate hydrogen protons. There are currently three definitions for acids and bases that entail how they behave when placed in solutions. Complexes of metal ions with water, ammonia, and hydroxide ion are examples. bases also react with certain metals, like zinc or aluminum, to produce hydrogen gas. What. Do Bases React With All Metals.
From readthescience.blogspot.com
READ THE SCIENCE 10.3The reaction of metals with acids Do Bases React With All Metals Most metals do not react with bases but zinc(zn) metal does. bases also react with certain metals, like zinc or aluminum, to produce hydrogen gas. They release energy to the surroundings and the released energy is associated with the. There are currently three definitions for acids and bases that entail how they behave when placed in solutions. all. Do Bases React With All Metals.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW Do Bases React With All Metals They release energy to the surroundings and the released energy is associated with the. Complexes of metal ions with water, ammonia, and hydroxide ion are examples. bases also react with certain metals, like zinc or aluminum, to produce hydrogen gas. all metal cations are potential lewis acids. some metal reacts with a base to form salts and. Do Bases React With All Metals.
From educationgrafts.z21.web.core.windows.net
What Do Bases React With Do Bases React With All Metals So what is an acid? Essentially, acids accept electron pairs and donate hydrogen protons. Most metals do not react with bases but zinc(zn) metal does. They release energy to the surroundings and the released energy is associated with the. all metal cations are potential lewis acids. bases do not react with metals in the way that acids do.. Do Bases React With All Metals.
From trivaldypiping.blogspot.com
Do Bases React With Metals To Produce Hydrogen Gas Do Bases React With All Metals What are acids and bases? bases do not react with metals in the way that acids do. Essentially, acids accept electron pairs and donate hydrogen protons. all metal cations are potential lewis acids. Complexes of metal ions with water, ammonia, and hydroxide ion are examples. some metal reacts with a base to form salts and hydrogen (h. Do Bases React With All Metals.
From www.slideserve.com
PPT Reactions of Metals PowerPoint Presentation, free download ID Do Bases React With All Metals bases do not react with metals in the way that acids do. They release energy to the surroundings and the released energy is associated with the. So what is an acid? some metal reacts with a base to form salts and hydrogen (h 2) gas. Essentially, acids accept electron pairs and donate hydrogen protons. Complexes of metal ions. Do Bases React With All Metals.
From sciencenotes.org
Metal Elements Science Notes and Projects Do Bases React With All Metals Essentially, acids accept electron pairs and donate hydrogen protons. So what is an acid? Most metals do not react with bases but zinc(zn) metal does. bases do not react with metals in the way that acids do. some metal reacts with a base to form salts and hydrogen (h 2) gas. They release energy to the surroundings and. Do Bases React With All Metals.
From www.toppr.com
How do acids and bases react with metals? Do Bases React With All Metals Most metals do not react with bases but zinc(zn) metal does. all metal cations are potential lewis acids. bases do not react with metals in the way that acids do. So what is an acid? Complexes of metal ions with water, ammonia, and hydroxide ion are examples. They release energy to the surroundings and the released energy is. Do Bases React With All Metals.
From www.youtube.com
Reaction of metals with water Class 10 Chemistry ICSE Board Do Bases React With All Metals Complexes of metal ions with water, ammonia, and hydroxide ion are examples. all metal cations are potential lewis acids. Bases react with acids to produce a salt and water. So what is an acid? There are currently three definitions for acids and bases that entail how they behave when placed in solutions. They release energy to the surroundings and. Do Bases React With All Metals.
From www.teachoo.com
Reaction of Metals and NonMetals with Acids Teachoo Concepts Do Bases React With All Metals bases do not react with metals in the way that acids do. some metal reacts with a base to form salts and hydrogen (h 2) gas. There are currently three definitions for acids and bases that entail how they behave when placed in solutions. all metal cations are potential lewis acids. They release energy to the surroundings. Do Bases React With All Metals.
From www.youtube.com
How do Bases react with Metals? Chapter 2 Class 10 Science CBSE Do Bases React With All Metals What are acids and bases? Essentially, acids accept electron pairs and donate hydrogen protons. There are currently three definitions for acids and bases that entail how they behave when placed in solutions. Most metals do not react with bases but zinc(zn) metal does. bases also react with certain metals, like zinc or aluminum, to produce hydrogen gas. Complexes of. Do Bases React With All Metals.
From tampasteel.com
What Is a Metal Alloy? Metal vs Metal Alloy Difference Tampa Steel Do Bases React With All Metals They release energy to the surroundings and the released energy is associated with the. Bases react with acids to produce a salt and water. bases also react with certain metals, like zinc or aluminum, to produce hydrogen gas. So what is an acid? What are acids and bases? Essentially, acids accept electron pairs and donate hydrogen protons. Complexes of. Do Bases React With All Metals.
From www.thesciencehive.co.uk
Acids, Bases and Salt Preparations (GCSE) — the science hive Do Bases React With All Metals all metal cations are potential lewis acids. Bases react with acids to produce a salt and water. They release energy to the surroundings and the released energy is associated with the. Most metals do not react with bases but zinc(zn) metal does. So what is an acid? Complexes of metal ions with water, ammonia, and hydroxide ion are examples.. Do Bases React With All Metals.
From www.youtube.com
Acids, Bases and Salts Class 10 Science How Do Acid and Base React Do Bases React With All Metals Most metals do not react with bases but zinc(zn) metal does. some metal reacts with a base to form salts and hydrogen (h 2) gas. Bases react with acids to produce a salt and water. Essentially, acids accept electron pairs and donate hydrogen protons. What are acids and bases? bases also react with certain metals, like zinc or. Do Bases React With All Metals.
From edurev.in
Why do all metals do not react with bases ? EduRev Class 10 Question Do Bases React With All Metals Complexes of metal ions with water, ammonia, and hydroxide ion are examples. all metal cations are potential lewis acids. So what is an acid? bases also react with certain metals, like zinc or aluminum, to produce hydrogen gas. bases do not react with metals in the way that acids do. There are currently three definitions for acids. Do Bases React With All Metals.
From learningpectose.z13.web.core.windows.net
How To Write Acid Base Reactions Do Bases React With All Metals some metal reacts with a base to form salts and hydrogen (h 2) gas. So what is an acid? Bases react with acids to produce a salt and water. Essentially, acids accept electron pairs and donate hydrogen protons. bases do not react with metals in the way that acids do. They release energy to the surroundings and the. Do Bases React With All Metals.
From www.chemicals.co.uk
A Level Chemistry Revision Physical Chemistry Acids And Bases Do Bases React With All Metals bases also react with certain metals, like zinc or aluminum, to produce hydrogen gas. Bases react with acids to produce a salt and water. Most metals do not react with bases but zinc(zn) metal does. Essentially, acids accept electron pairs and donate hydrogen protons. What are acids and bases? They release energy to the surroundings and the released energy. Do Bases React With All Metals.
From getrevising.co.uk
Topic 1 Atomic Structure and the periodic table Revision Cards in Do Bases React With All Metals bases do not react with metals in the way that acids do. all metal cations are potential lewis acids. Most metals do not react with bases but zinc(zn) metal does. What are acids and bases? bases also react with certain metals, like zinc or aluminum, to produce hydrogen gas. Complexes of metal ions with water, ammonia, and. Do Bases React With All Metals.
From www.goodscience.com.au
Metal Reactions Good Science Do Bases React With All Metals What are acids and bases? all metal cations are potential lewis acids. some metal reacts with a base to form salts and hydrogen (h 2) gas. There are currently three definitions for acids and bases that entail how they behave when placed in solutions. Most metals do not react with bases but zinc(zn) metal does. Essentially, acids accept. Do Bases React With All Metals.
From www.scribd.com
How Do Bases React With Metals PDF Do Bases React With All Metals bases do not react with metals in the way that acids do. Bases react with acids to produce a salt and water. There are currently three definitions for acids and bases that entail how they behave when placed in solutions. They release energy to the surroundings and the released energy is associated with the. Complexes of metal ions with. Do Bases React With All Metals.