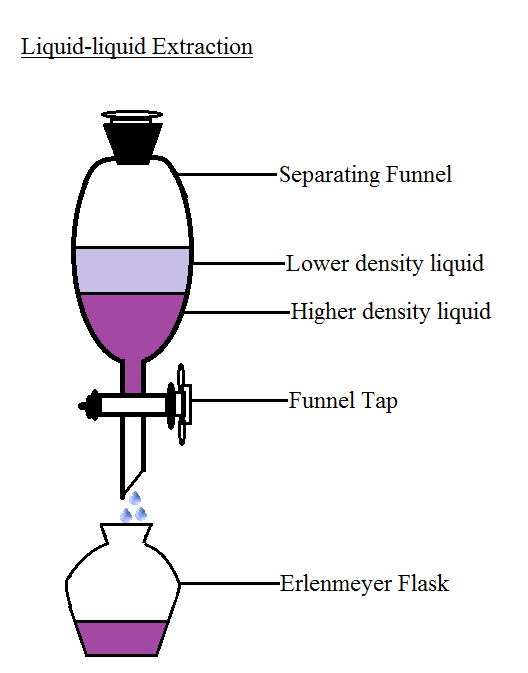
Solvent Extraction Lab Chemistry . In a multiple extraction procedure, a quantity of solvent is used to extract one layer (often the aqueous layer) multiple times in succession. It is useful for separating mixtures of compounds and removing. This technique uses two solvents which are immiscible, for example an organic solvent such as diethyl ether can be used to. If another extraction is to be done, return the bottom layer to the conical. Extraction is the process of transferring a substance to a solvent. Extraction is a frequently used technique to selectively transfer a compound of interested from one solvent to another. If the top layer is the desired layer, remove it from the conical vial using a fresh pipette into a clean container. The extraction is repeated two to three times, or.
from school.careers360.com
This technique uses two solvents which are immiscible, for example an organic solvent such as diethyl ether can be used to. It is useful for separating mixtures of compounds and removing. If another extraction is to be done, return the bottom layer to the conical. Extraction is a frequently used technique to selectively transfer a compound of interested from one solvent to another. The extraction is repeated two to three times, or. If the top layer is the desired layer, remove it from the conical vial using a fresh pipette into a clean container. In a multiple extraction procedure, a quantity of solvent is used to extract one layer (often the aqueous layer) multiple times in succession. Extraction is the process of transferring a substance to a solvent.
methods of separation Overview, Structure, Properties & Uses
Solvent Extraction Lab Chemistry In a multiple extraction procedure, a quantity of solvent is used to extract one layer (often the aqueous layer) multiple times in succession. Extraction is a frequently used technique to selectively transfer a compound of interested from one solvent to another. It is useful for separating mixtures of compounds and removing. The extraction is repeated two to three times, or. In a multiple extraction procedure, a quantity of solvent is used to extract one layer (often the aqueous layer) multiple times in succession. If another extraction is to be done, return the bottom layer to the conical. Extraction is the process of transferring a substance to a solvent. If the top layer is the desired layer, remove it from the conical vial using a fresh pipette into a clean container. This technique uses two solvents which are immiscible, for example an organic solvent such as diethyl ether can be used to.
From pubs.acs.org
A Colorful Solvent Extraction Demonstration for Teaching the Concept of Solvent Extraction Lab Chemistry The extraction is repeated two to three times, or. Extraction is the process of transferring a substance to a solvent. If another extraction is to be done, return the bottom layer to the conical. Extraction is a frequently used technique to selectively transfer a compound of interested from one solvent to another. This technique uses two solvents which are immiscible,. Solvent Extraction Lab Chemistry.
From eduinput.com
Solvent Extraction Types, Principle, uses Solvent Extraction Lab Chemistry It is useful for separating mixtures of compounds and removing. This technique uses two solvents which are immiscible, for example an organic solvent such as diethyl ether can be used to. Extraction is the process of transferring a substance to a solvent. In a multiple extraction procedure, a quantity of solvent is used to extract one layer (often the aqueous. Solvent Extraction Lab Chemistry.
From extractiongradesolvents.com
Modern Methods of Extraction ExtractionGradeSolvents Solvent Extraction Lab Chemistry Extraction is a frequently used technique to selectively transfer a compound of interested from one solvent to another. The extraction is repeated two to three times, or. This technique uses two solvents which are immiscible, for example an organic solvent such as diethyl ether can be used to. If another extraction is to be done, return the bottom layer to. Solvent Extraction Lab Chemistry.
From www.dreamstime.com
Chemical Extraction of Organic Compound from Water Solution To Organic Solvent Extraction Lab Chemistry It is useful for separating mixtures of compounds and removing. If another extraction is to be done, return the bottom layer to the conical. In a multiple extraction procedure, a quantity of solvent is used to extract one layer (often the aqueous layer) multiple times in succession. Extraction is a frequently used technique to selectively transfer a compound of interested. Solvent Extraction Lab Chemistry.
From www.pngegg.com
Chemistry Steam distillation Decantation Solvent in chemical reactions Solvent Extraction Lab Chemistry If another extraction is to be done, return the bottom layer to the conical. It is useful for separating mixtures of compounds and removing. Extraction is a frequently used technique to selectively transfer a compound of interested from one solvent to another. The extraction is repeated two to three times, or. If the top layer is the desired layer, remove. Solvent Extraction Lab Chemistry.
From www.mdpi.com
Water Free FullText LiquidLiquid Continuous Extraction and Solvent Extraction Lab Chemistry The extraction is repeated two to three times, or. In a multiple extraction procedure, a quantity of solvent is used to extract one layer (often the aqueous layer) multiple times in succession. Extraction is a frequently used technique to selectively transfer a compound of interested from one solvent to another. Extraction is the process of transferring a substance to a. Solvent Extraction Lab Chemistry.
From www.aurorabiomed.com
LiquidLiquid Extraction vs. SolidPhase Extraction Solvent Extraction Lab Chemistry Extraction is the process of transferring a substance to a solvent. If the top layer is the desired layer, remove it from the conical vial using a fresh pipette into a clean container. Extraction is a frequently used technique to selectively transfer a compound of interested from one solvent to another. In a multiple extraction procedure, a quantity of solvent. Solvent Extraction Lab Chemistry.
From studylibdiana.z13.web.core.windows.net
Organic Chemistry Extraction Flow Chart Solvent Extraction Lab Chemistry In a multiple extraction procedure, a quantity of solvent is used to extract one layer (often the aqueous layer) multiple times in succession. The extraction is repeated two to three times, or. Extraction is a frequently used technique to selectively transfer a compound of interested from one solvent to another. If another extraction is to be done, return the bottom. Solvent Extraction Lab Chemistry.
From www.youtube.com
solvent extraction by chemistry lover part 1 ch 2 lec 4 YouTube Solvent Extraction Lab Chemistry It is useful for separating mixtures of compounds and removing. In a multiple extraction procedure, a quantity of solvent is used to extract one layer (often the aqueous layer) multiple times in succession. Extraction is a frequently used technique to selectively transfer a compound of interested from one solvent to another. This technique uses two solvents which are immiscible, for. Solvent Extraction Lab Chemistry.
From chem.libretexts.org
7.20 AcidBase Extraction Chemistry LibreTexts Solvent Extraction Lab Chemistry Extraction is the process of transferring a substance to a solvent. This technique uses two solvents which are immiscible, for example an organic solvent such as diethyl ether can be used to. It is useful for separating mixtures of compounds and removing. Extraction is a frequently used technique to selectively transfer a compound of interested from one solvent to another.. Solvent Extraction Lab Chemistry.
From www.slideserve.com
PPT Solvent extraction PowerPoint Presentation, free download ID Solvent Extraction Lab Chemistry The extraction is repeated two to three times, or. It is useful for separating mixtures of compounds and removing. If another extraction is to be done, return the bottom layer to the conical. This technique uses two solvents which are immiscible, for example an organic solvent such as diethyl ether can be used to. In a multiple extraction procedure, a. Solvent Extraction Lab Chemistry.
From pubs.acs.org
Simultaneous Solvent Selection and Process Design for Continuous Solvent Extraction Lab Chemistry This technique uses two solvents which are immiscible, for example an organic solvent such as diethyl ether can be used to. If another extraction is to be done, return the bottom layer to the conical. In a multiple extraction procedure, a quantity of solvent is used to extract one layer (often the aqueous layer) multiple times in succession. Extraction is. Solvent Extraction Lab Chemistry.
From www.chegg.com
Solved Extraction Organic chemistry question about aqueous Solvent Extraction Lab Chemistry The extraction is repeated two to three times, or. If another extraction is to be done, return the bottom layer to the conical. This technique uses two solvents which are immiscible, for example an organic solvent such as diethyl ether can be used to. It is useful for separating mixtures of compounds and removing. Extraction is a frequently used technique. Solvent Extraction Lab Chemistry.
From mavink.com
Solvent Extraction Diagram Solvent Extraction Lab Chemistry In a multiple extraction procedure, a quantity of solvent is used to extract one layer (often the aqueous layer) multiple times in succession. It is useful for separating mixtures of compounds and removing. Extraction is a frequently used technique to selectively transfer a compound of interested from one solvent to another. If the top layer is the desired layer, remove. Solvent Extraction Lab Chemistry.
From eduinput.com
Solvent Extraction Types, Principle, uses Solvent Extraction Lab Chemistry If the top layer is the desired layer, remove it from the conical vial using a fresh pipette into a clean container. Extraction is the process of transferring a substance to a solvent. Extraction is a frequently used technique to selectively transfer a compound of interested from one solvent to another. In a multiple extraction procedure, a quantity of solvent. Solvent Extraction Lab Chemistry.
From chem.libretexts.org
4.6 StepbyStep Procedures For Extractions Chemistry LibreTexts Solvent Extraction Lab Chemistry In a multiple extraction procedure, a quantity of solvent is used to extract one layer (often the aqueous layer) multiple times in succession. Extraction is the process of transferring a substance to a solvent. This technique uses two solvents which are immiscible, for example an organic solvent such as diethyl ether can be used to. It is useful for separating. Solvent Extraction Lab Chemistry.
From study.com
Solvent Extraction Definition & Process Lesson Solvent Extraction Lab Chemistry Extraction is the process of transferring a substance to a solvent. The extraction is repeated two to three times, or. This technique uses two solvents which are immiscible, for example an organic solvent such as diethyl ether can be used to. Extraction is a frequently used technique to selectively transfer a compound of interested from one solvent to another. In. Solvent Extraction Lab Chemistry.
From chemistnotes.com
Soxhlet Extraction Principle, Extraction procedure, and Apparatus Solvent Extraction Lab Chemistry If another extraction is to be done, return the bottom layer to the conical. Extraction is the process of transferring a substance to a solvent. If the top layer is the desired layer, remove it from the conical vial using a fresh pipette into a clean container. This technique uses two solvents which are immiscible, for example an organic solvent. Solvent Extraction Lab Chemistry.
From simplesolvents.com
Solvent Extraction 101 Techniques, Applications, & Solvents Solvent Extraction Lab Chemistry In a multiple extraction procedure, a quantity of solvent is used to extract one layer (often the aqueous layer) multiple times in succession. This technique uses two solvents which are immiscible, for example an organic solvent such as diethyl ether can be used to. Extraction is the process of transferring a substance to a solvent. It is useful for separating. Solvent Extraction Lab Chemistry.
From pubs.acs.org
Nonaqueous Solvent Extraction for Enhanced Metal Separations Concept Solvent Extraction Lab Chemistry Extraction is the process of transferring a substance to a solvent. If another extraction is to be done, return the bottom layer to the conical. This technique uses two solvents which are immiscible, for example an organic solvent such as diethyl ether can be used to. Extraction is a frequently used technique to selectively transfer a compound of interested from. Solvent Extraction Lab Chemistry.
From www.slideshare.net
Extraction Solvent Extraction Lab Chemistry This technique uses two solvents which are immiscible, for example an organic solvent such as diethyl ether can be used to. If another extraction is to be done, return the bottom layer to the conical. If the top layer is the desired layer, remove it from the conical vial using a fresh pipette into a clean container. Extraction is a. Solvent Extraction Lab Chemistry.
From allaboutchemistry123.blogspot.com
Solvent extraction, principle, technique, types of extraction Solvent Extraction Lab Chemistry If the top layer is the desired layer, remove it from the conical vial using a fresh pipette into a clean container. Extraction is a frequently used technique to selectively transfer a compound of interested from one solvent to another. In a multiple extraction procedure, a quantity of solvent is used to extract one layer (often the aqueous layer) multiple. Solvent Extraction Lab Chemistry.
From www.researchgate.net
Solvent extraction system used for extraction of phospholipids from Solvent Extraction Lab Chemistry If another extraction is to be done, return the bottom layer to the conical. Extraction is the process of transferring a substance to a solvent. The extraction is repeated two to three times, or. If the top layer is the desired layer, remove it from the conical vial using a fresh pipette into a clean container. It is useful for. Solvent Extraction Lab Chemistry.
From www.sciencephoto.com
Scientist doing a solventbased extraction Stock Image C036/9020 Solvent Extraction Lab Chemistry Extraction is the process of transferring a substance to a solvent. The extraction is repeated two to three times, or. Extraction is a frequently used technique to selectively transfer a compound of interested from one solvent to another. It is useful for separating mixtures of compounds and removing. If the top layer is the desired layer, remove it from the. Solvent Extraction Lab Chemistry.
From www.slideshare.net
Extraction Solvent Extraction Lab Chemistry The extraction is repeated two to three times, or. If the top layer is the desired layer, remove it from the conical vial using a fresh pipette into a clean container. Extraction is a frequently used technique to selectively transfer a compound of interested from one solvent to another. In a multiple extraction procedure, a quantity of solvent is used. Solvent Extraction Lab Chemistry.
From www.youtube.com
ALevel PreLab Video for Using a Separating Funnel YouTube Solvent Extraction Lab Chemistry It is useful for separating mixtures of compounds and removing. The extraction is repeated two to three times, or. If another extraction is to be done, return the bottom layer to the conical. This technique uses two solvents which are immiscible, for example an organic solvent such as diethyl ether can be used to. Extraction is a frequently used technique. Solvent Extraction Lab Chemistry.
From chem.libretexts.org
4.6 StepbyStep Procedures For Extractions Chemistry LibreTexts Solvent Extraction Lab Chemistry The extraction is repeated two to three times, or. If the top layer is the desired layer, remove it from the conical vial using a fresh pipette into a clean container. Extraction is the process of transferring a substance to a solvent. In a multiple extraction procedure, a quantity of solvent is used to extract one layer (often the aqueous. Solvent Extraction Lab Chemistry.
From chem.libretexts.org
4.2 Overview of Extraction Chemistry LibreTexts Solvent Extraction Lab Chemistry Extraction is a frequently used technique to selectively transfer a compound of interested from one solvent to another. If the top layer is the desired layer, remove it from the conical vial using a fresh pipette into a clean container. If another extraction is to be done, return the bottom layer to the conical. In a multiple extraction procedure, a. Solvent Extraction Lab Chemistry.
From favpng.com
Laboratory Extraction Microwave Chemistry CEM Corporation Solvent In Solvent Extraction Lab Chemistry It is useful for separating mixtures of compounds and removing. If the top layer is the desired layer, remove it from the conical vial using a fresh pipette into a clean container. The extraction is repeated two to three times, or. In a multiple extraction procedure, a quantity of solvent is used to extract one layer (often the aqueous layer). Solvent Extraction Lab Chemistry.
From school.careers360.com
methods of separation Overview, Structure, Properties & Uses Solvent Extraction Lab Chemistry It is useful for separating mixtures of compounds and removing. Extraction is a frequently used technique to selectively transfer a compound of interested from one solvent to another. If the top layer is the desired layer, remove it from the conical vial using a fresh pipette into a clean container. Extraction is the process of transferring a substance to a. Solvent Extraction Lab Chemistry.
From chemistnotes.com
Solvent extraction Principle, easy process, application Chemistry Notes Solvent Extraction Lab Chemistry Extraction is a frequently used technique to selectively transfer a compound of interested from one solvent to another. Extraction is the process of transferring a substance to a solvent. It is useful for separating mixtures of compounds and removing. The extraction is repeated two to three times, or. This technique uses two solvents which are immiscible, for example an organic. Solvent Extraction Lab Chemistry.
From www.studocu.com
orgo lab 4Separating Acids and Neutral Compounds by Solvent Extraction Solvent Extraction Lab Chemistry In a multiple extraction procedure, a quantity of solvent is used to extract one layer (often the aqueous layer) multiple times in succession. If another extraction is to be done, return the bottom layer to the conical. This technique uses two solvents which are immiscible, for example an organic solvent such as diethyl ether can be used to. Extraction is. Solvent Extraction Lab Chemistry.
From www.alamy.com
Researcher. ASE. Accelerated solvent extraction. Extraction of organic Solvent Extraction Lab Chemistry Extraction is the process of transferring a substance to a solvent. In a multiple extraction procedure, a quantity of solvent is used to extract one layer (often the aqueous layer) multiple times in succession. If the top layer is the desired layer, remove it from the conical vial using a fresh pipette into a clean container. Extraction is a frequently. Solvent Extraction Lab Chemistry.
From www.coleparmer.ca
Plant Solvent Extraction Method Using Ethanol 3 Steps ColeParmer Canada Solvent Extraction Lab Chemistry This technique uses two solvents which are immiscible, for example an organic solvent such as diethyl ether can be used to. If the top layer is the desired layer, remove it from the conical vial using a fresh pipette into a clean container. It is useful for separating mixtures of compounds and removing. The extraction is repeated two to three. Solvent Extraction Lab Chemistry.
From www.youtube.com
Solvent Extraction with Example Chapter 2F.Sc Chemistry Part1 Solvent Extraction Lab Chemistry Extraction is the process of transferring a substance to a solvent. If another extraction is to be done, return the bottom layer to the conical. In a multiple extraction procedure, a quantity of solvent is used to extract one layer (often the aqueous layer) multiple times in succession. The extraction is repeated two to three times, or. If the top. Solvent Extraction Lab Chemistry.