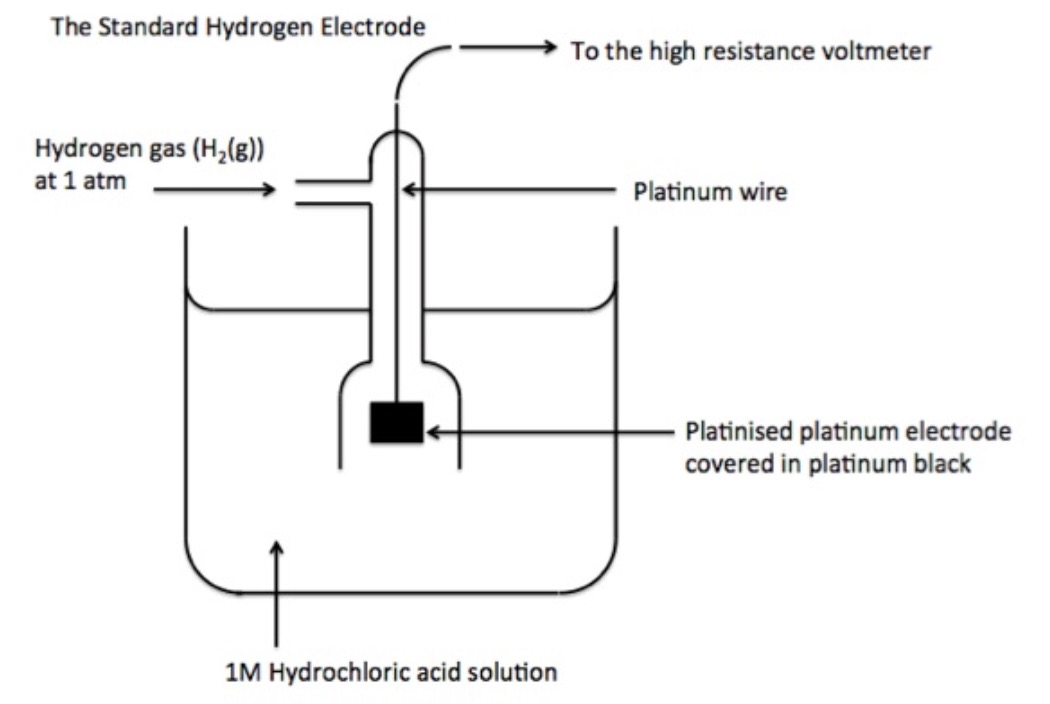
Standard Electrode Potential Videos . Interpret electrode potentials in terms of relative oxidant and reductant. As electrode potentials measure reduction capacity, an increased e°. Since the potential of the s.h.e. The standard cell potential is a measure of the driving force for a given redox reaction. Is zero, we define the standard electrode potential, \ ( {\mathcal {e}}^o\), of any other standard half. This video covers electrode potentials at standard conditions and introduces the standard electrode reduction potential table. A stronger oxidant exhibits greater standard electrode potential, e°. Describe and relate the definitions of electrode and cell potentials.
from classnotes.org.in
Is zero, we define the standard electrode potential, \ ( {\mathcal {e}}^o\), of any other standard half. A stronger oxidant exhibits greater standard electrode potential, e°. The standard cell potential is a measure of the driving force for a given redox reaction. Describe and relate the definitions of electrode and cell potentials. As electrode potentials measure reduction capacity, an increased e°. Interpret electrode potentials in terms of relative oxidant and reductant. Since the potential of the s.h.e. This video covers electrode potentials at standard conditions and introduces the standard electrode reduction potential table.
Electrode Potential and E.M.F. of a Galvanic Cell Chemistry, Class 12
Standard Electrode Potential Videos As electrode potentials measure reduction capacity, an increased e°. Is zero, we define the standard electrode potential, \ ( {\mathcal {e}}^o\), of any other standard half. Describe and relate the definitions of electrode and cell potentials. A stronger oxidant exhibits greater standard electrode potential, e°. Since the potential of the s.h.e. Interpret electrode potentials in terms of relative oxidant and reductant. As electrode potentials measure reduction capacity, an increased e°. This video covers electrode potentials at standard conditions and introduces the standard electrode reduction potential table. The standard cell potential is a measure of the driving force for a given redox reaction.
From byjus.com
6. The standard electrode potential E^° I2/I , E^° Br /Br2 and E^° Fe Standard Electrode Potential Videos Describe and relate the definitions of electrode and cell potentials. A stronger oxidant exhibits greater standard electrode potential, e°. This video covers electrode potentials at standard conditions and introduces the standard electrode reduction potential table. The standard cell potential is a measure of the driving force for a given redox reaction. As electrode potentials measure reduction capacity, an increased e°.. Standard Electrode Potential Videos.
From cbsencertsolutiononline.blogspot.com
CBSE NCERT SOLUTIONS The standard electrode potentials at 298 K Standard Electrode Potential Videos This video covers electrode potentials at standard conditions and introduces the standard electrode reduction potential table. Describe and relate the definitions of electrode and cell potentials. Since the potential of the s.h.e. As electrode potentials measure reduction capacity, an increased e°. The standard cell potential is a measure of the driving force for a given redox reaction. Interpret electrode potentials. Standard Electrode Potential Videos.
From mungfali.com
Table Of Standard Electrode Potentials Standard Electrode Potential Videos Describe and relate the definitions of electrode and cell potentials. This video covers electrode potentials at standard conditions and introduces the standard electrode reduction potential table. Since the potential of the s.h.e. A stronger oxidant exhibits greater standard electrode potential, e°. Is zero, we define the standard electrode potential, \ ( {\mathcal {e}}^o\), of any other standard half. The standard. Standard Electrode Potential Videos.
From dinorahiu-images.blogspot.com
Standard Potential Table / 18 4 Standard Electrode Potential Powerpoint Standard Electrode Potential Videos As electrode potentials measure reduction capacity, an increased e°. The standard cell potential is a measure of the driving force for a given redox reaction. Describe and relate the definitions of electrode and cell potentials. Is zero, we define the standard electrode potential, \ ( {\mathcal {e}}^o\), of any other standard half. Interpret electrode potentials in terms of relative oxidant. Standard Electrode Potential Videos.
From free-images.com
Free Images standard electrode potential zinc Standard Electrode Potential Videos The standard cell potential is a measure of the driving force for a given redox reaction. Interpret electrode potentials in terms of relative oxidant and reductant. Describe and relate the definitions of electrode and cell potentials. Since the potential of the s.h.e. As electrode potentials measure reduction capacity, an increased e°. A stronger oxidant exhibits greater standard electrode potential, e°.. Standard Electrode Potential Videos.
From www.youtube.com
12th Chemistry Ch8Part8Trends in the M3+/M2+ standard electrode Standard Electrode Potential Videos A stronger oxidant exhibits greater standard electrode potential, e°. As electrode potentials measure reduction capacity, an increased e°. Describe and relate the definitions of electrode and cell potentials. Interpret electrode potentials in terms of relative oxidant and reductant. Is zero, we define the standard electrode potential, \ ( {\mathcal {e}}^o\), of any other standard half. This video covers electrode potentials. Standard Electrode Potential Videos.
From www.substech.com
standard_electrode_potential.png [SubsTech] Standard Electrode Potential Videos Describe and relate the definitions of electrode and cell potentials. A stronger oxidant exhibits greater standard electrode potential, e°. This video covers electrode potentials at standard conditions and introduces the standard electrode reduction potential table. As electrode potentials measure reduction capacity, an increased e°. Is zero, we define the standard electrode potential, \ ( {\mathcal {e}}^o\), of any other standard. Standard Electrode Potential Videos.
From youtube.com
19.1.4 Predict whether a reaction will be spontaneous using standard Standard Electrode Potential Videos The standard cell potential is a measure of the driving force for a given redox reaction. Describe and relate the definitions of electrode and cell potentials. A stronger oxidant exhibits greater standard electrode potential, e°. Is zero, we define the standard electrode potential, \ ( {\mathcal {e}}^o\), of any other standard half. As electrode potentials measure reduction capacity, an increased. Standard Electrode Potential Videos.
From www.slideserve.com
PPT Chapter 20 Electrochemistry PowerPoint Presentation, free Standard Electrode Potential Videos Interpret electrode potentials in terms of relative oxidant and reductant. As electrode potentials measure reduction capacity, an increased e°. Is zero, we define the standard electrode potential, \ ( {\mathcal {e}}^o\), of any other standard half. This video covers electrode potentials at standard conditions and introduces the standard electrode reduction potential table. Describe and relate the definitions of electrode and. Standard Electrode Potential Videos.
From question.pandai.org
Standard Electrode Potential Standard Electrode Potential Videos Interpret electrode potentials in terms of relative oxidant and reductant. A stronger oxidant exhibits greater standard electrode potential, e°. As electrode potentials measure reduction capacity, an increased e°. Is zero, we define the standard electrode potential, \ ( {\mathcal {e}}^o\), of any other standard half. The standard cell potential is a measure of the driving force for a given redox. Standard Electrode Potential Videos.
From www.youtube.com
APSC132 lecture 5 03 Standard Electrode Potential YouTube Standard Electrode Potential Videos Is zero, we define the standard electrode potential, \ ( {\mathcal {e}}^o\), of any other standard half. As electrode potentials measure reduction capacity, an increased e°. Describe and relate the definitions of electrode and cell potentials. Since the potential of the s.h.e. This video covers electrode potentials at standard conditions and introduces the standard electrode reduction potential table. A stronger. Standard Electrode Potential Videos.
From www.flinnsci.ca
Standard Reduction Potential Chart Flinn Scientific Standard Electrode Potential Videos Interpret electrode potentials in terms of relative oxidant and reductant. This video covers electrode potentials at standard conditions and introduces the standard electrode reduction potential table. A stronger oxidant exhibits greater standard electrode potential, e°. Is zero, we define the standard electrode potential, \ ( {\mathcal {e}}^o\), of any other standard half. Describe and relate the definitions of electrode and. Standard Electrode Potential Videos.
From www.pveducation.org
Standard Potential PVEducation Standard Electrode Potential Videos This video covers electrode potentials at standard conditions and introduces the standard electrode reduction potential table. Is zero, we define the standard electrode potential, \ ( {\mathcal {e}}^o\), of any other standard half. Interpret electrode potentials in terms of relative oxidant and reductant. The standard cell potential is a measure of the driving force for a given redox reaction. A. Standard Electrode Potential Videos.
From users.highland.edu
Standard Potentials Standard Electrode Potential Videos Describe and relate the definitions of electrode and cell potentials. Is zero, we define the standard electrode potential, \ ( {\mathcal {e}}^o\), of any other standard half. As electrode potentials measure reduction capacity, an increased e°. A stronger oxidant exhibits greater standard electrode potential, e°. Since the potential of the s.h.e. This video covers electrode potentials at standard conditions and. Standard Electrode Potential Videos.
From mungfali.com
Standard Electrode Potential Table Standard Electrode Potential Videos A stronger oxidant exhibits greater standard electrode potential, e°. Describe and relate the definitions of electrode and cell potentials. Is zero, we define the standard electrode potential, \ ( {\mathcal {e}}^o\), of any other standard half. This video covers electrode potentials at standard conditions and introduces the standard electrode reduction potential table. As electrode potentials measure reduction capacity, an increased. Standard Electrode Potential Videos.
From www.youtube.com
19.1 Calculate cell potentials using standard electrode potentials [HL Standard Electrode Potential Videos Interpret electrode potentials in terms of relative oxidant and reductant. Describe and relate the definitions of electrode and cell potentials. Since the potential of the s.h.e. As electrode potentials measure reduction capacity, an increased e°. The standard cell potential is a measure of the driving force for a given redox reaction. A stronger oxidant exhibits greater standard electrode potential, e°.. Standard Electrode Potential Videos.
From chem.libretexts.org
Standard Potentials Chemistry LibreTexts Standard Electrode Potential Videos Interpret electrode potentials in terms of relative oxidant and reductant. This video covers electrode potentials at standard conditions and introduces the standard electrode reduction potential table. The standard cell potential is a measure of the driving force for a given redox reaction. A stronger oxidant exhibits greater standard electrode potential, e°. Is zero, we define the standard electrode potential, \. Standard Electrode Potential Videos.
From meteonmendez.blogspot.com
Standard Electrode Potential Table MeteonMendez Standard Electrode Potential Videos Interpret electrode potentials in terms of relative oxidant and reductant. Describe and relate the definitions of electrode and cell potentials. Is zero, we define the standard electrode potential, \ ( {\mathcal {e}}^o\), of any other standard half. A stronger oxidant exhibits greater standard electrode potential, e°. This video covers electrode potentials at standard conditions and introduces the standard electrode reduction. Standard Electrode Potential Videos.
From classnotes.org.in
Electrode Potential and E.M.F. of a Galvanic Cell Chemistry, Class 12 Standard Electrode Potential Videos This video covers electrode potentials at standard conditions and introduces the standard electrode reduction potential table. Since the potential of the s.h.e. As electrode potentials measure reduction capacity, an increased e°. Interpret electrode potentials in terms of relative oxidant and reductant. Is zero, we define the standard electrode potential, \ ( {\mathcal {e}}^o\), of any other standard half. The standard. Standard Electrode Potential Videos.
From app.pandai.org
Standard Electrode Potential Standard Electrode Potential Videos The standard cell potential is a measure of the driving force for a given redox reaction. Interpret electrode potentials in terms of relative oxidant and reductant. Is zero, we define the standard electrode potential, \ ( {\mathcal {e}}^o\), of any other standard half. Since the potential of the s.h.e. This video covers electrode potentials at standard conditions and introduces the. Standard Electrode Potential Videos.
From testbook.com
Standard Electrode PotentialLearn Definition,Formula,Conditions Standard Electrode Potential Videos Interpret electrode potentials in terms of relative oxidant and reductant. The standard cell potential is a measure of the driving force for a given redox reaction. Since the potential of the s.h.e. Is zero, we define the standard electrode potential, \ ( {\mathcal {e}}^o\), of any other standard half. A stronger oxidant exhibits greater standard electrode potential, e°. Describe and. Standard Electrode Potential Videos.
From www.slideserve.com
PPT Chapter 17 Electrochemistry PowerPoint Presentation, free Standard Electrode Potential Videos Interpret electrode potentials in terms of relative oxidant and reductant. This video covers electrode potentials at standard conditions and introduces the standard electrode reduction potential table. The standard cell potential is a measure of the driving force for a given redox reaction. As electrode potentials measure reduction capacity, an increased e°. Since the potential of the s.h.e. Describe and relate. Standard Electrode Potential Videos.
From byjus.com
Standard electrode potentials are Fe2+/Fe; E° = 0.44 volts Fe3+/Fe2+; E Standard Electrode Potential Videos Since the potential of the s.h.e. Interpret electrode potentials in terms of relative oxidant and reductant. As electrode potentials measure reduction capacity, an increased e°. The standard cell potential is a measure of the driving force for a given redox reaction. A stronger oxidant exhibits greater standard electrode potential, e°. Is zero, we define the standard electrode potential, \ (. Standard Electrode Potential Videos.
From www.tpsearchtool.com
Standard Reduction Potential Charts For Chemistry Images Standard Electrode Potential Videos A stronger oxidant exhibits greater standard electrode potential, e°. Describe and relate the definitions of electrode and cell potentials. As electrode potentials measure reduction capacity, an increased e°. This video covers electrode potentials at standard conditions and introduces the standard electrode reduction potential table. Since the potential of the s.h.e. Interpret electrode potentials in terms of relative oxidant and reductant.. Standard Electrode Potential Videos.
From www.youtube.com
Using Standard Electrode Potentials YouTube Standard Electrode Potential Videos Describe and relate the definitions of electrode and cell potentials. Is zero, we define the standard electrode potential, \ ( {\mathcal {e}}^o\), of any other standard half. The standard cell potential is a measure of the driving force for a given redox reaction. As electrode potentials measure reduction capacity, an increased e°. Interpret electrode potentials in terms of relative oxidant. Standard Electrode Potential Videos.
From 2012books.lardbucket.org
Standard Potentials Standard Electrode Potential Videos This video covers electrode potentials at standard conditions and introduces the standard electrode reduction potential table. As electrode potentials measure reduction capacity, an increased e°. Describe and relate the definitions of electrode and cell potentials. Since the potential of the s.h.e. A stronger oxidant exhibits greater standard electrode potential, e°. Interpret electrode potentials in terms of relative oxidant and reductant.. Standard Electrode Potential Videos.
From www.youtube.com
12th Chemistry Ch8Part7Trends in the M2+/M standard electrode Standard Electrode Potential Videos A stronger oxidant exhibits greater standard electrode potential, e°. As electrode potentials measure reduction capacity, an increased e°. Is zero, we define the standard electrode potential, \ ( {\mathcal {e}}^o\), of any other standard half. Describe and relate the definitions of electrode and cell potentials. Since the potential of the s.h.e. This video covers electrode potentials at standard conditions and. Standard Electrode Potential Videos.
From dxofmrhhh.blob.core.windows.net
Standard Electrode Potential Pdf at Arthur Baker blog Standard Electrode Potential Videos Interpret electrode potentials in terms of relative oxidant and reductant. The standard cell potential is a measure of the driving force for a given redox reaction. As electrode potentials measure reduction capacity, an increased e°. This video covers electrode potentials at standard conditions and introduces the standard electrode reduction potential table. Is zero, we define the standard electrode potential, \. Standard Electrode Potential Videos.
From question.pandai.org
Standard Electrode Potential Standard Electrode Potential Videos A stronger oxidant exhibits greater standard electrode potential, e°. This video covers electrode potentials at standard conditions and introduces the standard electrode reduction potential table. Interpret electrode potentials in terms of relative oxidant and reductant. Since the potential of the s.h.e. The standard cell potential is a measure of the driving force for a given redox reaction. Is zero, we. Standard Electrode Potential Videos.
From www.academia.edu
(PDF) Standard Electrode Potential Aubriecia Lim Academia.edu Standard Electrode Potential Videos Interpret electrode potentials in terms of relative oxidant and reductant. This video covers electrode potentials at standard conditions and introduces the standard electrode reduction potential table. As electrode potentials measure reduction capacity, an increased e°. A stronger oxidant exhibits greater standard electrode potential, e°. Describe and relate the definitions of electrode and cell potentials. The standard cell potential is a. Standard Electrode Potential Videos.
From dxofmrhhh.blob.core.windows.net
Standard Electrode Potential Pdf at Arthur Baker blog Standard Electrode Potential Videos Since the potential of the s.h.e. Describe and relate the definitions of electrode and cell potentials. This video covers electrode potentials at standard conditions and introduces the standard electrode reduction potential table. The standard cell potential is a measure of the driving force for a given redox reaction. Interpret electrode potentials in terms of relative oxidant and reductant. As electrode. Standard Electrode Potential Videos.
From mungfali.com
Electrode Potential Series Standard Electrode Potential Videos Is zero, we define the standard electrode potential, \ ( {\mathcal {e}}^o\), of any other standard half. Since the potential of the s.h.e. Describe and relate the definitions of electrode and cell potentials. Interpret electrode potentials in terms of relative oxidant and reductant. This video covers electrode potentials at standard conditions and introduces the standard electrode reduction potential table. A. Standard Electrode Potential Videos.
From www.youtube.com
19.1 Standard electrode potential (HL) YouTube Standard Electrode Potential Videos This video covers electrode potentials at standard conditions and introduces the standard electrode reduction potential table. Interpret electrode potentials in terms of relative oxidant and reductant. As electrode potentials measure reduction capacity, an increased e°. A stronger oxidant exhibits greater standard electrode potential, e°. Since the potential of the s.h.e. Describe and relate the definitions of electrode and cell potentials.. Standard Electrode Potential Videos.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Standard Electrode Potential Videos Is zero, we define the standard electrode potential, \ ( {\mathcal {e}}^o\), of any other standard half. Interpret electrode potentials in terms of relative oxidant and reductant. This video covers electrode potentials at standard conditions and introduces the standard electrode reduction potential table. Describe and relate the definitions of electrode and cell potentials. Since the potential of the s.h.e. As. Standard Electrode Potential Videos.
From www.youtube.com
Determination of Standard Electrode potential of 'Zn and Cu Standard Electrode Potential Videos Is zero, we define the standard electrode potential, \ ( {\mathcal {e}}^o\), of any other standard half. This video covers electrode potentials at standard conditions and introduces the standard electrode reduction potential table. Since the potential of the s.h.e. Interpret electrode potentials in terms of relative oxidant and reductant. As electrode potentials measure reduction capacity, an increased e°. The standard. Standard Electrode Potential Videos.