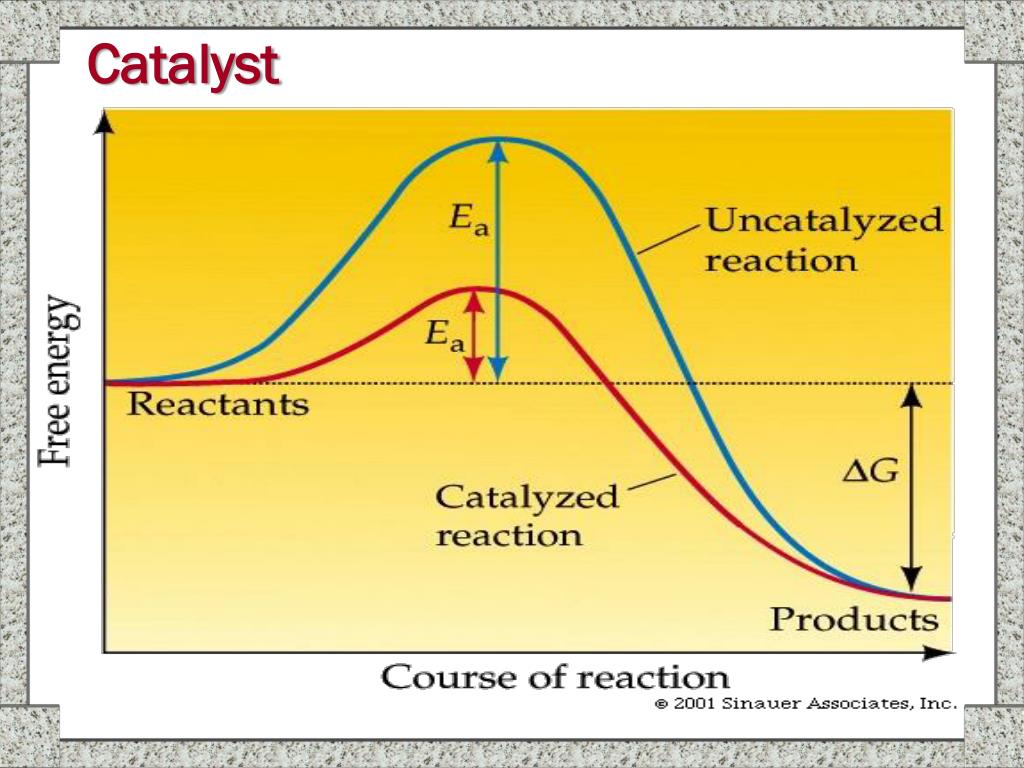
Catalyst Rate Of Reaction Discussion . Another way in which the rate of reaction could be measured for. Explain how adding a catalyst increases the rate of reaction. Different substances catalyse different reactions. Rate of reaction is a measure of how fast a reaction takes place. I can describe what a catalyst does and explain how it affects the rate of reaction in terms of activation energy, and justify its use for a. This page describes and explains the way that adding a catalyst affects the rate of a reaction. A catalyst provides an alternative pathway for the reaction that has a lower. Catalysts are substances which speed up the rate of a reaction without themselves being altered or consumed in the reaction. In industry, chemists control rates of reaction to ensure the production is. Only a very small mass of catalyst is needed to increase the rate of a reaction. Describe how changing the temperature, concentration of a reactant, or surface area of a reaction affects the rate of a. However, not all reactions have suitable catalysts. The rate of the reaction can be calculated by drawing a tangent to the line of best fit over the first 30 seconds. It assumes that you are already familiar with basic ideas about the collision theory of reaction. The mass of a catalyst at the beginning and end of a reaction.
from www.slideserve.com
Explain how adding a catalyst increases the rate of reaction. Different substances catalyse different reactions. The rate of the reaction can be calculated by drawing a tangent to the line of best fit over the first 30 seconds. Catalysts are substances which speed up the rate of a reaction without themselves being altered or consumed in the reaction. I can describe what a catalyst does and explain how it affects the rate of reaction in terms of activation energy, and justify its use for a. This page describes and explains the way that adding a catalyst affects the rate of a reaction. In industry, chemists control rates of reaction to ensure the production is. Rate of reaction is a measure of how fast a reaction takes place. However, not all reactions have suitable catalysts. A catalyst provides an alternative pathway for the reaction that has a lower.
PPT Rates of Reactions PowerPoint Presentation, free download ID
Catalyst Rate Of Reaction Discussion However, not all reactions have suitable catalysts. It assumes that you are already familiar with basic ideas about the collision theory of reaction. However, not all reactions have suitable catalysts. I can describe what a catalyst does and explain how it affects the rate of reaction in terms of activation energy, and justify its use for a. A catalyst provides an alternative pathway for the reaction that has a lower. This page describes and explains the way that adding a catalyst affects the rate of a reaction. Another way in which the rate of reaction could be measured for. In industry, chemists control rates of reaction to ensure the production is. The rate of the reaction can be calculated by drawing a tangent to the line of best fit over the first 30 seconds. Explain how adding a catalyst increases the rate of reaction. Describe how changing the temperature, concentration of a reactant, or surface area of a reaction affects the rate of a. Only a very small mass of catalyst is needed to increase the rate of a reaction. Different substances catalyse different reactions. The mass of a catalyst at the beginning and end of a reaction. Rate of reaction is a measure of how fast a reaction takes place. Catalysts are substances which speed up the rate of a reaction without themselves being altered or consumed in the reaction.
From www.researchgate.net
Initial reaction rate versus catalyst loading; molar ratio acetic acid Catalyst Rate Of Reaction Discussion However, not all reactions have suitable catalysts. In industry, chemists control rates of reaction to ensure the production is. Explain how adding a catalyst increases the rate of reaction. This page describes and explains the way that adding a catalyst affects the rate of a reaction. The rate of the reaction can be calculated by drawing a tangent to the. Catalyst Rate Of Reaction Discussion.
From www.numerade.com
SOLVEDDoes a catalyst increase reaction rate by the same means as a Catalyst Rate Of Reaction Discussion Only a very small mass of catalyst is needed to increase the rate of a reaction. A catalyst provides an alternative pathway for the reaction that has a lower. In industry, chemists control rates of reaction to ensure the production is. Rate of reaction is a measure of how fast a reaction takes place. Describe how changing the temperature, concentration. Catalyst Rate Of Reaction Discussion.
From answerhappy.com
A catalyst is a substance that increases the rate of reaction but does Catalyst Rate Of Reaction Discussion Only a very small mass of catalyst is needed to increase the rate of a reaction. In industry, chemists control rates of reaction to ensure the production is. Rate of reaction is a measure of how fast a reaction takes place. A catalyst provides an alternative pathway for the reaction that has a lower. This page describes and explains the. Catalyst Rate Of Reaction Discussion.
From studyfullfarrior.z19.web.core.windows.net
Rates Of Reaction And Energy Changes Catalyst Rate Of Reaction Discussion In industry, chemists control rates of reaction to ensure the production is. Different substances catalyse different reactions. Only a very small mass of catalyst is needed to increase the rate of a reaction. It assumes that you are already familiar with basic ideas about the collision theory of reaction. Describe how changing the temperature, concentration of a reactant, or surface. Catalyst Rate Of Reaction Discussion.
From www.slideserve.com
PPT Rates of Reactions PowerPoint Presentation, free download ID Catalyst Rate Of Reaction Discussion In industry, chemists control rates of reaction to ensure the production is. A catalyst provides an alternative pathway for the reaction that has a lower. Another way in which the rate of reaction could be measured for. Describe how changing the temperature, concentration of a reactant, or surface area of a reaction affects the rate of a. Explain how adding. Catalyst Rate Of Reaction Discussion.
From www.youtube.com
A Catalyst and the Rate of Reaction YouTube Catalyst Rate Of Reaction Discussion The mass of a catalyst at the beginning and end of a reaction. However, not all reactions have suitable catalysts. This page describes and explains the way that adding a catalyst affects the rate of a reaction. Another way in which the rate of reaction could be measured for. Different substances catalyse different reactions. It assumes that you are already. Catalyst Rate Of Reaction Discussion.
From www.meritnation.com
Illustrate graphically the effect of a catalyst on rate of a reaction Catalyst Rate Of Reaction Discussion This page describes and explains the way that adding a catalyst affects the rate of a reaction. Catalysts are substances which speed up the rate of a reaction without themselves being altered or consumed in the reaction. However, not all reactions have suitable catalysts. Different substances catalyse different reactions. Only a very small mass of catalyst is needed to increase. Catalyst Rate Of Reaction Discussion.
From www.slideserve.com
PPT Rates of reactions PowerPoint Presentation, free download ID Catalyst Rate Of Reaction Discussion The mass of a catalyst at the beginning and end of a reaction. However, not all reactions have suitable catalysts. I can describe what a catalyst does and explain how it affects the rate of reaction in terms of activation energy, and justify its use for a. Catalysts are substances which speed up the rate of a reaction without themselves. Catalyst Rate Of Reaction Discussion.
From wiringfixunripping.z21.web.core.windows.net
Reaction Energy Diagram With Catalyst Catalyst Rate Of Reaction Discussion The mass of a catalyst at the beginning and end of a reaction. The rate of the reaction can be calculated by drawing a tangent to the line of best fit over the first 30 seconds. Only a very small mass of catalyst is needed to increase the rate of a reaction. Another way in which the rate of reaction. Catalyst Rate Of Reaction Discussion.
From www.edukate.me
Explain The Effect of Catalyst on the Rate of Reaction A catalyst Catalyst Rate Of Reaction Discussion Only a very small mass of catalyst is needed to increase the rate of a reaction. However, not all reactions have suitable catalysts. It assumes that you are already familiar with basic ideas about the collision theory of reaction. In industry, chemists control rates of reaction to ensure the production is. This page describes and explains the way that adding. Catalyst Rate Of Reaction Discussion.
From www.researchgate.net
Reaction coordinate diagram showing the working principle of a catalyst Catalyst Rate Of Reaction Discussion Explain how adding a catalyst increases the rate of reaction. Another way in which the rate of reaction could be measured for. Different substances catalyse different reactions. A catalyst provides an alternative pathway for the reaction that has a lower. Only a very small mass of catalyst is needed to increase the rate of a reaction. Rate of reaction is. Catalyst Rate Of Reaction Discussion.
From www.slideserve.com
PPT RATES OF REACTION A guide for GCSE students PowerPoint Catalyst Rate Of Reaction Discussion However, not all reactions have suitable catalysts. Catalysts are substances which speed up the rate of a reaction without themselves being altered or consumed in the reaction. Explain how adding a catalyst increases the rate of reaction. I can describe what a catalyst does and explain how it affects the rate of reaction in terms of activation energy, and justify. Catalyst Rate Of Reaction Discussion.
From jackwestin.com
Rate Processes Catalysts Rate Processes In Chemical Reactions Catalyst Rate Of Reaction Discussion Rate of reaction is a measure of how fast a reaction takes place. Explain how adding a catalyst increases the rate of reaction. In industry, chemists control rates of reaction to ensure the production is. Describe how changing the temperature, concentration of a reactant, or surface area of a reaction affects the rate of a. A catalyst provides an alternative. Catalyst Rate Of Reaction Discussion.
From ppt-online.org
Rates of reaction презентация онлайн Catalyst Rate Of Reaction Discussion Different substances catalyse different reactions. In industry, chemists control rates of reaction to ensure the production is. The mass of a catalyst at the beginning and end of a reaction. Describe how changing the temperature, concentration of a reactant, or surface area of a reaction affects the rate of a. Rate of reaction is a measure of how fast a. Catalyst Rate Of Reaction Discussion.
From www.mometrix.com
What is a Catalyst? Chemistry Review (Video) Catalyst Rate Of Reaction Discussion In industry, chemists control rates of reaction to ensure the production is. Only a very small mass of catalyst is needed to increase the rate of a reaction. The rate of the reaction can be calculated by drawing a tangent to the line of best fit over the first 30 seconds. The mass of a catalyst at the beginning and. Catalyst Rate Of Reaction Discussion.
From www.youtube.com
Catalyst Affects Reaction Rate Energy Diagram with a Catalyst YouTube Catalyst Rate Of Reaction Discussion A catalyst provides an alternative pathway for the reaction that has a lower. Catalysts are substances which speed up the rate of a reaction without themselves being altered or consumed in the reaction. The rate of the reaction can be calculated by drawing a tangent to the line of best fit over the first 30 seconds. Rate of reaction is. Catalyst Rate Of Reaction Discussion.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Catalyst Rate Of Reaction Discussion It assumes that you are already familiar with basic ideas about the collision theory of reaction. Describe how changing the temperature, concentration of a reactant, or surface area of a reaction affects the rate of a. Different substances catalyse different reactions. However, not all reactions have suitable catalysts. In industry, chemists control rates of reaction to ensure the production is.. Catalyst Rate Of Reaction Discussion.
From www.slideserve.com
PPT Reaction Rates and Equilibrium PowerPoint Presentation, free Catalyst Rate Of Reaction Discussion However, not all reactions have suitable catalysts. The mass of a catalyst at the beginning and end of a reaction. It assumes that you are already familiar with basic ideas about the collision theory of reaction. I can describe what a catalyst does and explain how it affects the rate of reaction in terms of activation energy, and justify its. Catalyst Rate Of Reaction Discussion.
From www.scribd.com
Chemistry, chapter 10, Rate of Reaction Catalysis Reaction Rate Catalyst Rate Of Reaction Discussion It assumes that you are already familiar with basic ideas about the collision theory of reaction. I can describe what a catalyst does and explain how it affects the rate of reaction in terms of activation energy, and justify its use for a. Catalysts are substances which speed up the rate of a reaction without themselves being altered or consumed. Catalyst Rate Of Reaction Discussion.
From studylib.net
Catalysts and Reaction Rate Catalyst Rate Of Reaction Discussion Different substances catalyse different reactions. I can describe what a catalyst does and explain how it affects the rate of reaction in terms of activation energy, and justify its use for a. In industry, chemists control rates of reaction to ensure the production is. It assumes that you are already familiar with basic ideas about the collision theory of reaction.. Catalyst Rate Of Reaction Discussion.
From www.slideserve.com
PPT Factors that Affect Rate of Reaction PowerPoint Presentation Catalyst Rate Of Reaction Discussion Another way in which the rate of reaction could be measured for. This page describes and explains the way that adding a catalyst affects the rate of a reaction. A catalyst provides an alternative pathway for the reaction that has a lower. Only a very small mass of catalyst is needed to increase the rate of a reaction. Explain how. Catalyst Rate Of Reaction Discussion.
From www.slideserve.com
PPT Reaction Rates 2 PowerPoint Presentation, free download ID3493687 Catalyst Rate Of Reaction Discussion Different substances catalyse different reactions. I can describe what a catalyst does and explain how it affects the rate of reaction in terms of activation energy, and justify its use for a. Describe how changing the temperature, concentration of a reactant, or surface area of a reaction affects the rate of a. The mass of a catalyst at the beginning. Catalyst Rate Of Reaction Discussion.
From www.slideshare.net
1.4 rate of reaction(1.2d)...biology Catalyst Rate Of Reaction Discussion This page describes and explains the way that adding a catalyst affects the rate of a reaction. Rate of reaction is a measure of how fast a reaction takes place. Only a very small mass of catalyst is needed to increase the rate of a reaction. In industry, chemists control rates of reaction to ensure the production is. Catalysts are. Catalyst Rate Of Reaction Discussion.
From www.youtube.com
How Catalysts Affect Rate Of Reaction GCSE Chemistry Catalyst Rate Of Reaction Discussion The mass of a catalyst at the beginning and end of a reaction. Rate of reaction is a measure of how fast a reaction takes place. In industry, chemists control rates of reaction to ensure the production is. This page describes and explains the way that adding a catalyst affects the rate of a reaction. Different substances catalyse different reactions.. Catalyst Rate Of Reaction Discussion.
From www.sasadoctor.com
Rate of reaction chemistry igcse Catalyst Rate Of Reaction Discussion Catalysts are substances which speed up the rate of a reaction without themselves being altered or consumed in the reaction. The mass of a catalyst at the beginning and end of a reaction. I can describe what a catalyst does and explain how it affects the rate of reaction in terms of activation energy, and justify its use for a.. Catalyst Rate Of Reaction Discussion.
From www.youtube.com
Effect of Catalyst on Rate of Reaction Chemical Chemistry Catalyst Rate Of Reaction Discussion Different substances catalyse different reactions. This page describes and explains the way that adding a catalyst affects the rate of a reaction. However, not all reactions have suitable catalysts. Explain how adding a catalyst increases the rate of reaction. I can describe what a catalyst does and explain how it affects the rate of reaction in terms of activation energy,. Catalyst Rate Of Reaction Discussion.
From slideplayer.com
Catalysts Rates of Reactions. ppt download Catalyst Rate Of Reaction Discussion It assumes that you are already familiar with basic ideas about the collision theory of reaction. Describe how changing the temperature, concentration of a reactant, or surface area of a reaction affects the rate of a. In industry, chemists control rates of reaction to ensure the production is. The rate of the reaction can be calculated by drawing a tangent. Catalyst Rate Of Reaction Discussion.
From www.youtube.com
Factors Affecting the Rate of Reaction Catalyst Rate of Reaction Catalyst Rate Of Reaction Discussion Different substances catalyse different reactions. The rate of the reaction can be calculated by drawing a tangent to the line of best fit over the first 30 seconds. Explain how adding a catalyst increases the rate of reaction. The mass of a catalyst at the beginning and end of a reaction. However, not all reactions have suitable catalysts. Only a. Catalyst Rate Of Reaction Discussion.
From slideplayer.com
Chemical Reactions and Collision Theory ppt download Catalyst Rate Of Reaction Discussion I can describe what a catalyst does and explain how it affects the rate of reaction in terms of activation energy, and justify its use for a. It assumes that you are already familiar with basic ideas about the collision theory of reaction. Rate of reaction is a measure of how fast a reaction takes place. Another way in which. Catalyst Rate Of Reaction Discussion.
From www.slideserve.com
PPT Factors Affecting the Rate of a Chemical Reaction PowerPoint Catalyst Rate Of Reaction Discussion This page describes and explains the way that adding a catalyst affects the rate of a reaction. The rate of the reaction can be calculated by drawing a tangent to the line of best fit over the first 30 seconds. Catalysts are substances which speed up the rate of a reaction without themselves being altered or consumed in the reaction.. Catalyst Rate Of Reaction Discussion.
From slideplayer.com
Catalysts Rates of Reactions. ppt download Catalyst Rate Of Reaction Discussion Catalysts are substances which speed up the rate of a reaction without themselves being altered or consumed in the reaction. In industry, chemists control rates of reaction to ensure the production is. The rate of the reaction can be calculated by drawing a tangent to the line of best fit over the first 30 seconds. Different substances catalyse different reactions.. Catalyst Rate Of Reaction Discussion.
From www.tes.com
Catalysts on the Rate of Reaction KS4 AQA Chemistry Teaching Resources Catalyst Rate Of Reaction Discussion The rate of the reaction can be calculated by drawing a tangent to the line of best fit over the first 30 seconds. It assumes that you are already familiar with basic ideas about the collision theory of reaction. I can describe what a catalyst does and explain how it affects the rate of reaction in terms of activation energy,. Catalyst Rate Of Reaction Discussion.
From www.expii.com
Rate of Reaction (Enzymes) — Role & Importance Expii Catalyst Rate Of Reaction Discussion A catalyst provides an alternative pathway for the reaction that has a lower. This page describes and explains the way that adding a catalyst affects the rate of a reaction. Another way in which the rate of reaction could be measured for. Describe how changing the temperature, concentration of a reactant, or surface area of a reaction affects the rate. Catalyst Rate Of Reaction Discussion.
From www.nagwa.com
Question Video Understanding How Catalysts Affect the Rates and the Catalyst Rate Of Reaction Discussion This page describes and explains the way that adding a catalyst affects the rate of a reaction. I can describe what a catalyst does and explain how it affects the rate of reaction in terms of activation energy, and justify its use for a. Rate of reaction is a measure of how fast a reaction takes place. It assumes that. Catalyst Rate Of Reaction Discussion.
From study.com
Effect of Catalysts on Rates of Reaction Lesson Catalyst Rate Of Reaction Discussion Rate of reaction is a measure of how fast a reaction takes place. The mass of a catalyst at the beginning and end of a reaction. However, not all reactions have suitable catalysts. Different substances catalyse different reactions. This page describes and explains the way that adding a catalyst affects the rate of a reaction. In industry, chemists control rates. Catalyst Rate Of Reaction Discussion.