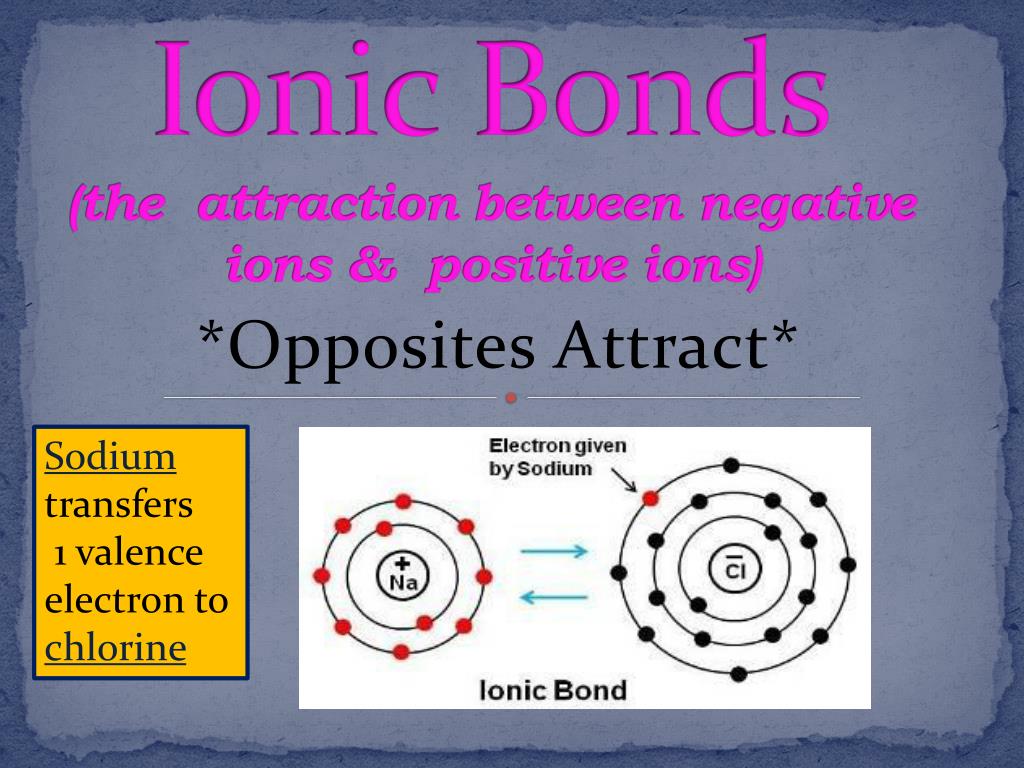
Is Sodium Ion Positive Or Negative . In this article, we will explore and compare the. Ionic formulas balance the total positive and negative charges. Neutral chlorine atom on left has 17 protons and 17 electrons. Sodium can exist in two forms: This means they still have 11 protons, but now have only 10. As a neutral atom (na) or as a positively charged ion (na+). Sodium atoms always lose one electron when they become ions. A sodium atom loses one electron to form a sodium ion. Ionic compounds have positive ions and negative ions. Revise the difference between ionic, covalent and metallic bonding, and understand how to interpret dot and cross diagrams. The sodium ion still has 11 protons (11 positive charges) but now only 10 electrons ( 10 negative charges). The sodium ion has an extra positive charge, shown by the + sign.
from www.slideserve.com
Sodium can exist in two forms: Revise the difference between ionic, covalent and metallic bonding, and understand how to interpret dot and cross diagrams. This means they still have 11 protons, but now have only 10. Ionic compounds have positive ions and negative ions. In this article, we will explore and compare the. Sodium atoms always lose one electron when they become ions. A sodium atom loses one electron to form a sodium ion. As a neutral atom (na) or as a positively charged ion (na+). The sodium ion still has 11 protons (11 positive charges) but now only 10 electrons ( 10 negative charges). The sodium ion has an extra positive charge, shown by the + sign.
PPT Ionic Bonds (the attraction between negative ions & positive ions
Is Sodium Ion Positive Or Negative The sodium ion has an extra positive charge, shown by the + sign. A sodium atom loses one electron to form a sodium ion. Sodium can exist in two forms: In this article, we will explore and compare the. The sodium ion has an extra positive charge, shown by the + sign. Revise the difference between ionic, covalent and metallic bonding, and understand how to interpret dot and cross diagrams. The sodium ion still has 11 protons (11 positive charges) but now only 10 electrons ( 10 negative charges). Neutral chlorine atom on left has 17 protons and 17 electrons. As a neutral atom (na) or as a positively charged ion (na+). This means they still have 11 protons, but now have only 10. Ionic formulas balance the total positive and negative charges. Sodium atoms always lose one electron when they become ions. Ionic compounds have positive ions and negative ions.
From chem.libretexts.org
2.6 Molecular and Ionic Compounds Chemistry LibreTexts Is Sodium Ion Positive Or Negative This means they still have 11 protons, but now have only 10. Ionic formulas balance the total positive and negative charges. In this article, we will explore and compare the. As a neutral atom (na) or as a positively charged ion (na+). A sodium atom loses one electron to form a sodium ion. Neutral chlorine atom on left has 17. Is Sodium Ion Positive Or Negative.
From alevelbiology.co.uk
Ions Types, Summary, Classification & Facts Is Sodium Ion Positive Or Negative Sodium atoms always lose one electron when they become ions. Revise the difference between ionic, covalent and metallic bonding, and understand how to interpret dot and cross diagrams. Ionic compounds have positive ions and negative ions. Neutral chlorine atom on left has 17 protons and 17 electrons. In this article, we will explore and compare the. The sodium ion still. Is Sodium Ion Positive Or Negative.
From www.slideserve.com
PPT Ionic Bonds (the attraction between negative ions & positive ions Is Sodium Ion Positive Or Negative Ionic formulas balance the total positive and negative charges. In this article, we will explore and compare the. A sodium atom loses one electron to form a sodium ion. Ionic compounds have positive ions and negative ions. Sodium can exist in two forms: As a neutral atom (na) or as a positively charged ion (na+). This means they still have. Is Sodium Ion Positive Or Negative.
From spmchemistry.blog.onlinetuition.com.my
Formation of Ion SPM Chemistry Is Sodium Ion Positive Or Negative Ionic compounds have positive ions and negative ions. The sodium ion has an extra positive charge, shown by the + sign. The sodium ion still has 11 protons (11 positive charges) but now only 10 electrons ( 10 negative charges). Sodium atoms always lose one electron when they become ions. Revise the difference between ionic, covalent and metallic bonding, and. Is Sodium Ion Positive Or Negative.
From openbooks.lib.msu.edu
Membrane Potential Foundations of Neuroscience Is Sodium Ion Positive Or Negative A sodium atom loses one electron to form a sodium ion. Sodium can exist in two forms: Revise the difference between ionic, covalent and metallic bonding, and understand how to interpret dot and cross diagrams. The sodium ion has an extra positive charge, shown by the + sign. Sodium atoms always lose one electron when they become ions. Neutral chlorine. Is Sodium Ion Positive Or Negative.
From wou.edu
CH150 Chapter 3 Ions and Ionic Compounds Chemistry Is Sodium Ion Positive Or Negative Ionic formulas balance the total positive and negative charges. Neutral chlorine atom on left has 17 protons and 17 electrons. Sodium can exist in two forms: In this article, we will explore and compare the. The sodium ion has an extra positive charge, shown by the + sign. This means they still have 11 protons, but now have only 10.. Is Sodium Ion Positive Or Negative.
From chem.libretexts.org
Ionic Solids Chemistry LibreTexts Is Sodium Ion Positive Or Negative This means they still have 11 protons, but now have only 10. A sodium atom loses one electron to form a sodium ion. As a neutral atom (na) or as a positively charged ion (na+). Neutral chlorine atom on left has 17 protons and 17 electrons. The sodium ion still has 11 protons (11 positive charges) but now only 10. Is Sodium Ion Positive Or Negative.
From www.chemistryland.com
Lab 11 Is Sodium Ion Positive Or Negative In this article, we will explore and compare the. The sodium ion still has 11 protons (11 positive charges) but now only 10 electrons ( 10 negative charges). As a neutral atom (na) or as a positively charged ion (na+). Revise the difference between ionic, covalent and metallic bonding, and understand how to interpret dot and cross diagrams. Sodium atoms. Is Sodium Ion Positive Or Negative.
From www.albert.io
Solubility Rules The Ultimate Guide to AP® Chemistry Albert.io Is Sodium Ion Positive Or Negative As a neutral atom (na) or as a positively charged ion (na+). Ionic compounds have positive ions and negative ions. Revise the difference between ionic, covalent and metallic bonding, and understand how to interpret dot and cross diagrams. Sodium can exist in two forms: Ionic formulas balance the total positive and negative charges. The sodium ion still has 11 protons. Is Sodium Ion Positive Or Negative.
From www.youtube.com
The sodium atom YouTube Is Sodium Ion Positive Or Negative This means they still have 11 protons, but now have only 10. Ionic compounds have positive ions and negative ions. As a neutral atom (na) or as a positively charged ion (na+). In this article, we will explore and compare the. Ionic formulas balance the total positive and negative charges. The sodium ion has an extra positive charge, shown by. Is Sodium Ion Positive Or Negative.
From general.chemistrysteps.com
Electron Configurations of Ions Chemistry Steps Is Sodium Ion Positive Or Negative Sodium atoms always lose one electron when they become ions. Sodium can exist in two forms: A sodium atom loses one electron to form a sodium ion. This means they still have 11 protons, but now have only 10. The sodium ion has an extra positive charge, shown by the + sign. Neutral chlorine atom on left has 17 protons. Is Sodium Ion Positive Or Negative.
From www.chegg.com
Solved Positive ion Negative ion Name Formula Sodium Is Sodium Ion Positive Or Negative The sodium ion has an extra positive charge, shown by the + sign. Neutral chlorine atom on left has 17 protons and 17 electrons. The sodium ion still has 11 protons (11 positive charges) but now only 10 electrons ( 10 negative charges). Ionic compounds have positive ions and negative ions. As a neutral atom (na) or as a positively. Is Sodium Ion Positive Or Negative.
From www.shutterstock.com
3,538 Sodium Atom Images, Stock Photos & Vectors Shutterstock Is Sodium Ion Positive Or Negative In this article, we will explore and compare the. The sodium ion still has 11 protons (11 positive charges) but now only 10 electrons ( 10 negative charges). Revise the difference between ionic, covalent and metallic bonding, and understand how to interpret dot and cross diagrams. Ionic compounds have positive ions and negative ions. As a neutral atom (na) or. Is Sodium Ion Positive Or Negative.
From www.slideserve.com
PPT Neurons and Synapses PowerPoint Presentation, free download ID Is Sodium Ion Positive Or Negative Sodium atoms always lose one electron when they become ions. As a neutral atom (na) or as a positively charged ion (na+). Revise the difference between ionic, covalent and metallic bonding, and understand how to interpret dot and cross diagrams. In this article, we will explore and compare the. Neutral chlorine atom on left has 17 protons and 17 electrons.. Is Sodium Ion Positive Or Negative.
From brainly.in
When NaCl dissolves in water, aqueous Na+ and Cl− ions result. The Is Sodium Ion Positive Or Negative The sodium ion still has 11 protons (11 positive charges) but now only 10 electrons ( 10 negative charges). Neutral chlorine atom on left has 17 protons and 17 electrons. This means they still have 11 protons, but now have only 10. Ionic formulas balance the total positive and negative charges. In this article, we will explore and compare the.. Is Sodium Ion Positive Or Negative.
From derekcarrsavvy-chemist.blogspot.com
savvychemist GCSE OCR Gateway Chemistry C2.2 di Bonding and the Is Sodium Ion Positive Or Negative A sodium atom loses one electron to form a sodium ion. Ionic compounds have positive ions and negative ions. This means they still have 11 protons, but now have only 10. In this article, we will explore and compare the. Sodium atoms always lose one electron when they become ions. The sodium ion still has 11 protons (11 positive charges). Is Sodium Ion Positive Or Negative.
From owlcation.com
Chemical Bonding How Do Atoms Combine? What Are the Forces That Bind Is Sodium Ion Positive Or Negative In this article, we will explore and compare the. As a neutral atom (na) or as a positively charged ion (na+). Sodium can exist in two forms: Ionic formulas balance the total positive and negative charges. Ionic compounds have positive ions and negative ions. Sodium atoms always lose one electron when they become ions. A sodium atom loses one electron. Is Sodium Ion Positive Or Negative.
From sciencenotes.org
Cations and Anions Definitions, Examples, and Differences Is Sodium Ion Positive Or Negative The sodium ion still has 11 protons (11 positive charges) but now only 10 electrons ( 10 negative charges). The sodium ion has an extra positive charge, shown by the + sign. This means they still have 11 protons, but now have only 10. Ionic formulas balance the total positive and negative charges. A sodium atom loses one electron to. Is Sodium Ion Positive Or Negative.
From www.youtube.com
How to find Protons & Electrons for the Sodium ion (Na+) YouTube Is Sodium Ion Positive Or Negative Sodium can exist in two forms: In this article, we will explore and compare the. The sodium ion still has 11 protons (11 positive charges) but now only 10 electrons ( 10 negative charges). Revise the difference between ionic, covalent and metallic bonding, and understand how to interpret dot and cross diagrams. The sodium ion has an extra positive charge,. Is Sodium Ion Positive Or Negative.
From courses.lumenlearning.com
3.4 Ionic Nomenclature The Basics of General, Organic, and Biological Is Sodium Ion Positive Or Negative Sodium can exist in two forms: Sodium atoms always lose one electron when they become ions. Ionic formulas balance the total positive and negative charges. In this article, we will explore and compare the. The sodium ion has an extra positive charge, shown by the + sign. The sodium ion still has 11 protons (11 positive charges) but now only. Is Sodium Ion Positive Or Negative.
From www.pinterest.com.au
Na+ Electron Configuration (Sodium Ion) Electron configuration Is Sodium Ion Positive Or Negative A sodium atom loses one electron to form a sodium ion. The sodium ion still has 11 protons (11 positive charges) but now only 10 electrons ( 10 negative charges). Sodium atoms always lose one electron when they become ions. This means they still have 11 protons, but now have only 10. The sodium ion has an extra positive charge,. Is Sodium Ion Positive Or Negative.
From www.chemistrystudent.com
Ionic Bonding (ALevel) ChemistryStudent Is Sodium Ion Positive Or Negative A sodium atom loses one electron to form a sodium ion. Revise the difference between ionic, covalent and metallic bonding, and understand how to interpret dot and cross diagrams. Sodium can exist in two forms: The sodium ion has an extra positive charge, shown by the + sign. In this article, we will explore and compare the. This means they. Is Sodium Ion Positive Or Negative.
From www.getbodysmart.com
Ions Cations and Anions GetBodySmart Is Sodium Ion Positive Or Negative The sodium ion has an extra positive charge, shown by the + sign. Ionic compounds have positive ions and negative ions. Ionic formulas balance the total positive and negative charges. A sodium atom loses one electron to form a sodium ion. Revise the difference between ionic, covalent and metallic bonding, and understand how to interpret dot and cross diagrams. Neutral. Is Sodium Ion Positive Or Negative.
From www.slideserve.com
PPT Giant Ionic Structures PowerPoint Presentation, free download Is Sodium Ion Positive Or Negative Sodium atoms always lose one electron when they become ions. As a neutral atom (na) or as a positively charged ion (na+). Sodium can exist in two forms: A sodium atom loses one electron to form a sodium ion. Revise the difference between ionic, covalent and metallic bonding, and understand how to interpret dot and cross diagrams. The sodium ion. Is Sodium Ion Positive Or Negative.
From www.sciencenewsforstudents.org
Explainer Ions and radicals in our world Science News for Students Is Sodium Ion Positive Or Negative This means they still have 11 protons, but now have only 10. Neutral chlorine atom on left has 17 protons and 17 electrons. As a neutral atom (na) or as a positively charged ion (na+). The sodium ion still has 11 protons (11 positive charges) but now only 10 electrons ( 10 negative charges). Ionic formulas balance the total positive. Is Sodium Ion Positive Or Negative.
From www.pinterest.com
What are the Characteristics of Electron, Proton and Neutron A Plus Is Sodium Ion Positive Or Negative The sodium ion has an extra positive charge, shown by the + sign. Neutral chlorine atom on left has 17 protons and 17 electrons. Sodium atoms always lose one electron when they become ions. Ionic compounds have positive ions and negative ions. Sodium can exist in two forms: Ionic formulas balance the total positive and negative charges. A sodium atom. Is Sodium Ion Positive Or Negative.
From www.slideserve.com
PPT KS4 Chemistry PowerPoint Presentation, free download ID1787201 Is Sodium Ion Positive Or Negative A sodium atom loses one electron to form a sodium ion. Revise the difference between ionic, covalent and metallic bonding, and understand how to interpret dot and cross diagrams. As a neutral atom (na) or as a positively charged ion (na+). Neutral chlorine atom on left has 17 protons and 17 electrons. Sodium can exist in two forms: This means. Is Sodium Ion Positive Or Negative.
From www.dreamstime.com
Anions and Cations for Example Sodium and Chlorine Atoms. Stock Vector Is Sodium Ion Positive Or Negative Revise the difference between ionic, covalent and metallic bonding, and understand how to interpret dot and cross diagrams. Neutral chlorine atom on left has 17 protons and 17 electrons. As a neutral atom (na) or as a positively charged ion (na+). The sodium ion still has 11 protons (11 positive charges) but now only 10 electrons ( 10 negative charges).. Is Sodium Ion Positive Or Negative.
From periodictable.me
Sodium Electron Configuration (Na) with Orbital Diagram Is Sodium Ion Positive Or Negative Neutral chlorine atom on left has 17 protons and 17 electrons. Revise the difference between ionic, covalent and metallic bonding, and understand how to interpret dot and cross diagrams. Ionic formulas balance the total positive and negative charges. As a neutral atom (na) or as a positively charged ion (na+). The sodium ion has an extra positive charge, shown by. Is Sodium Ion Positive Or Negative.
From www.nagwa.com
Lesson Video Ions Nagwa Is Sodium Ion Positive Or Negative The sodium ion has an extra positive charge, shown by the + sign. As a neutral atom (na) or as a positively charged ion (na+). Ionic formulas balance the total positive and negative charges. This means they still have 11 protons, but now have only 10. Revise the difference between ionic, covalent and metallic bonding, and understand how to interpret. Is Sodium Ion Positive Or Negative.
From www.youtube.com
Difference between Na and Na+ (Sodium atom vs Sodium ion) YouTube Is Sodium Ion Positive Or Negative Sodium atoms always lose one electron when they become ions. The sodium ion still has 11 protons (11 positive charges) but now only 10 electrons ( 10 negative charges). In this article, we will explore and compare the. A sodium atom loses one electron to form a sodium ion. Neutral chlorine atom on left has 17 protons and 17 electrons.. Is Sodium Ion Positive Or Negative.
From www.newtondesk.com
sodium electron configuration Newton Desk Is Sodium Ion Positive Or Negative The sodium ion still has 11 protons (11 positive charges) but now only 10 electrons ( 10 negative charges). In this article, we will explore and compare the. The sodium ion has an extra positive charge, shown by the + sign. This means they still have 11 protons, but now have only 10. Revise the difference between ionic, covalent and. Is Sodium Ion Positive Or Negative.
From derekcarrsavvy-chemist.blogspot.com
savvychemist GCSE OCR Gateway Chemistry C2.2 di Bonding and the Is Sodium Ion Positive Or Negative The sodium ion has an extra positive charge, shown by the + sign. Ionic compounds have positive ions and negative ions. Revise the difference between ionic, covalent and metallic bonding, and understand how to interpret dot and cross diagrams. The sodium ion still has 11 protons (11 positive charges) but now only 10 electrons ( 10 negative charges). Neutral chlorine. Is Sodium Ion Positive Or Negative.
From www.youtube.com
Number of Ions in Na2CO3 Sodium carbonate YouTube Is Sodium Ion Positive Or Negative The sodium ion has an extra positive charge, shown by the + sign. Revise the difference between ionic, covalent and metallic bonding, and understand how to interpret dot and cross diagrams. The sodium ion still has 11 protons (11 positive charges) but now only 10 electrons ( 10 negative charges). This means they still have 11 protons, but now have. Is Sodium Ion Positive Or Negative.
From www.slideserve.com
PPT How do atoms form ions? PowerPoint Presentation, free download Is Sodium Ion Positive Or Negative The sodium ion still has 11 protons (11 positive charges) but now only 10 electrons ( 10 negative charges). This means they still have 11 protons, but now have only 10. Ionic compounds have positive ions and negative ions. Neutral chlorine atom on left has 17 protons and 17 electrons. Revise the difference between ionic, covalent and metallic bonding, and. Is Sodium Ion Positive Or Negative.