Titration Mass Formula . C t is the titrant. The concentration of analyte may then be calculated using the following formula: [7] c a = (c t × v t × m)/v a. The titration formula is used to determine the concentration of a particular substance in a solution. The mass of a mole of a substance is its relative mass expressed in grams. C a is the analyte concentration in molarity; If the titrant and analyte have a 1:1 mole ratio, the formula is molarity (m) of the acid x volume (v) of the acid = molarity (m) of the base x volume (v) of. In all cases, the reaction chosen for the analysis must. Provide pupils with a few different worked examples of the same. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. Ma is the molarity of the acid, while mb is the molarity of the base. The volumes of acids and alkali solutions that react with each other can be measured by. Va and vb are the volumes of the acid and base, respectively.
from www.tes.com
Ma is the molarity of the acid, while mb is the molarity of the base. Provide pupils with a few different worked examples of the same. Va and vb are the volumes of the acid and base, respectively. The titration formula is used to determine the concentration of a particular substance in a solution. The volumes of acids and alkali solutions that react with each other can be measured by. [7] c a = (c t × v t × m)/v a. The concentration of analyte may then be calculated using the following formula: C t is the titrant. The mass of a mole of a substance is its relative mass expressed in grams. If the titrant and analyte have a 1:1 mole ratio, the formula is molarity (m) of the acid x volume (v) of the acid = molarity (m) of the base x volume (v) of.
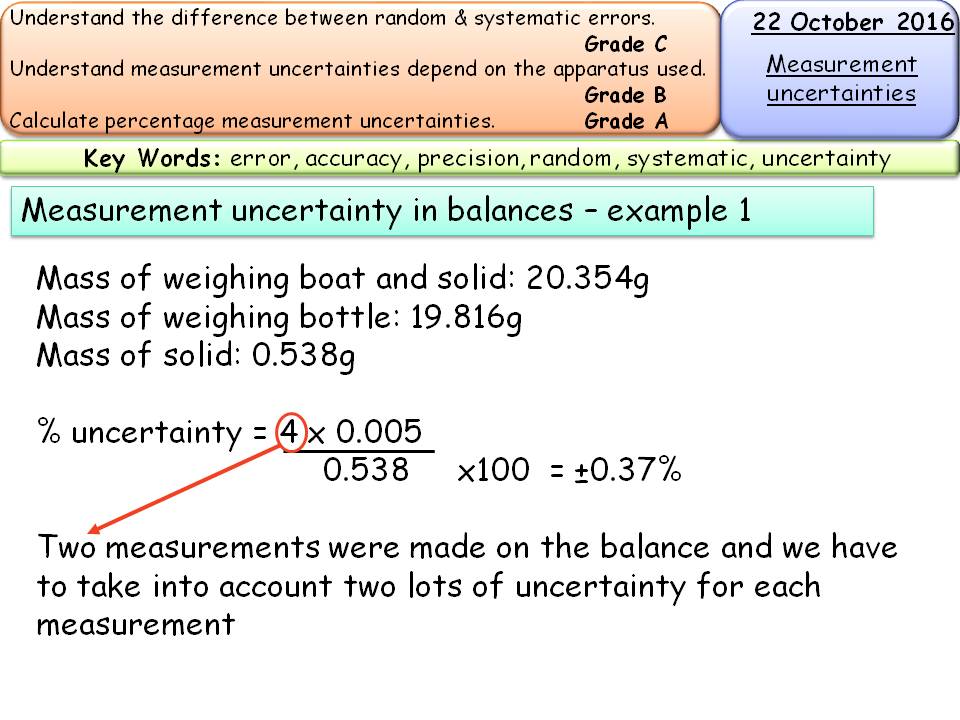
Measurement calculations/uncertainty for Titrations AS Chemistry
Titration Mass Formula C t is the titrant. If the titrant and analyte have a 1:1 mole ratio, the formula is molarity (m) of the acid x volume (v) of the acid = molarity (m) of the base x volume (v) of. In all cases, the reaction chosen for the analysis must. The concentration of analyte may then be calculated using the following formula: The volumes of acids and alkali solutions that react with each other can be measured by. Ma is the molarity of the acid, while mb is the molarity of the base. Va and vb are the volumes of the acid and base, respectively. [7] c a = (c t × v t × m)/v a. C a is the analyte concentration in molarity; C t is the titrant. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. The titration formula is used to determine the concentration of a particular substance in a solution. The mass of a mole of a substance is its relative mass expressed in grams. Provide pupils with a few different worked examples of the same.
From www.ck12.org
Titration (Calculations) Example 2 ( Video ) Chemistry CK12 Titration Mass Formula The volumes of acids and alkali solutions that react with each other can be measured by. C t is the titrant. If the titrant and analyte have a 1:1 mole ratio, the formula is molarity (m) of the acid x volume (v) of the acid = molarity (m) of the base x volume (v) of. In all cases, the reaction. Titration Mass Formula.
From theedge.com.hk
Chemistry How To Titration The Edge Titration Mass Formula A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. The concentration of analyte may then be calculated using the following formula: [7] c a = (c t × v t × m)/v a. C a is the analyte. Titration Mass Formula.
From mungfali.com
Acid Base Titration Calculation Titration Mass Formula The titration formula is used to determine the concentration of a particular substance in a solution. Va and vb are the volumes of the acid and base, respectively. In all cases, the reaction chosen for the analysis must. If the titrant and analyte have a 1:1 mole ratio, the formula is molarity (m) of the acid x volume (v) of. Titration Mass Formula.
From www.tes.com
Measurement calculations/uncertainty for Titrations AS Chemistry Titration Mass Formula [7] c a = (c t × v t × m)/v a. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. In all cases, the reaction chosen for the analysis must. The mass of a mole of a. Titration Mass Formula.
From www.slideserve.com
PPT Calculating concentrations PowerPoint Presentation, free download Titration Mass Formula Ma is the molarity of the acid, while mb is the molarity of the base. If the titrant and analyte have a 1:1 mole ratio, the formula is molarity (m) of the acid x volume (v) of the acid = molarity (m) of the base x volume (v) of. A titration is a volumetric technique in which a solution of. Titration Mass Formula.
From chem4three.blogspot.com
CHEMISTRY 11 TITRATIONS Titration Mass Formula The titration formula is used to determine the concentration of a particular substance in a solution. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. In all cases, the reaction chosen for the analysis must. If the titrant. Titration Mass Formula.
From www.youtube.com
How to Do Titration Calculations // HSC Chemistry YouTube Titration Mass Formula The titration formula is used to determine the concentration of a particular substance in a solution. The mass of a mole of a substance is its relative mass expressed in grams. Ma is the molarity of the acid, while mb is the molarity of the base. A titration is a volumetric technique in which a solution of one reactant (the. Titration Mass Formula.
From www.showme.com
Titration calculation Science, Chemistry, Physical Chemistry ShowMe Titration Mass Formula The concentration of analyte may then be calculated using the following formula: The mass of a mole of a substance is its relative mass expressed in grams. Provide pupils with a few different worked examples of the same. [7] c a = (c t × v t × m)/v a. If the titrant and analyte have a 1:1 mole ratio,. Titration Mass Formula.
From www.youtube.com
Titration Calculations National 5 Chemistry Lesson 5 YouTube Titration Mass Formula The mass of a mole of a substance is its relative mass expressed in grams. The titration formula is used to determine the concentration of a particular substance in a solution. Va and vb are the volumes of the acid and base, respectively. In all cases, the reaction chosen for the analysis must. If the titrant and analyte have a. Titration Mass Formula.
From www.youtube.com
Acid Base Titration Curves pH Calculations YouTube Titration Mass Formula A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. Va and vb are the volumes of the acid and base, respectively. Ma is the molarity of the acid, while mb is the molarity of the base. The concentration. Titration Mass Formula.
From www.slideserve.com
PPT TITRATION PowerPoint Presentation, free download ID1459481 Titration Mass Formula The volumes of acids and alkali solutions that react with each other can be measured by. Provide pupils with a few different worked examples of the same. Ma is the molarity of the acid, while mb is the molarity of the base. The concentration of analyte may then be calculated using the following formula: The mass of a mole of. Titration Mass Formula.
From www.slideserve.com
PPT Volumetric ANALYSIS/TITRATION PowerPoint Presentation, free Titration Mass Formula C t is the titrant. In all cases, the reaction chosen for the analysis must. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. The titration formula is used to determine the concentration of a particular substance in. Titration Mass Formula.
From studylib.net
Molarity, moles & mass and Volumetric titration calculations Titration Mass Formula Va and vb are the volumes of the acid and base, respectively. Ma is the molarity of the acid, while mb is the molarity of the base. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. [7] c. Titration Mass Formula.
From www.youtube.com
AcidBase Titration Equivalence Point YouTube Titration Mass Formula Va and vb are the volumes of the acid and base, respectively. C a is the analyte concentration in molarity; The titration formula is used to determine the concentration of a particular substance in a solution. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant. Titration Mass Formula.
From missmeyerscience.blogspot.com
Miss Meyer's Science Site Titration Calculations Titration Mass Formula The concentration of analyte may then be calculated using the following formula: Va and vb are the volumes of the acid and base, respectively. The volumes of acids and alkali solutions that react with each other can be measured by. Provide pupils with a few different worked examples of the same. The mass of a mole of a substance is. Titration Mass Formula.
From www.youtube.com
Acid Base Titration Problems, Basic Introduction, Calculations Titration Mass Formula The concentration of analyte may then be calculated using the following formula: The titration formula is used to determine the concentration of a particular substance in a solution. The volumes of acids and alkali solutions that react with each other can be measured by. The mass of a mole of a substance is its relative mass expressed in grams. C. Titration Mass Formula.
From www.slideserve.com
PPT Neutralization Reactions PowerPoint Presentation, free download Titration Mass Formula [7] c a = (c t × v t × m)/v a. C t is the titrant. The titration formula is used to determine the concentration of a particular substance in a solution. Provide pupils with a few different worked examples of the same. The mass of a mole of a substance is its relative mass expressed in grams. Ma. Titration Mass Formula.
From www.toppr.com
The formula mass of an acid is 82 amu. In a titration, 100 cm^3 of a Titration Mass Formula The concentration of analyte may then be calculated using the following formula: The volumes of acids and alkali solutions that react with each other can be measured by. [7] c a = (c t × v t × m)/v a. Provide pupils with a few different worked examples of the same. C a is the analyte concentration in molarity; Ma. Titration Mass Formula.
From www.youtube.com
Learn how to calculate the average mass of aspirin in tablets using a Titration Mass Formula The concentration of analyte may then be calculated using the following formula: Va and vb are the volumes of the acid and base, respectively. If the titrant and analyte have a 1:1 mole ratio, the formula is molarity (m) of the acid x volume (v) of the acid = molarity (m) of the base x volume (v) of. The volumes. Titration Mass Formula.
From www.youtube.com
How to Calculate Concentration using Titration Example in Chemistry Titration Mass Formula A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. Provide pupils with a few different worked examples of the same. If the titrant and analyte have a 1:1 mole ratio, the formula is molarity (m) of the acid. Titration Mass Formula.
From www.vrogue.co
Titration Concentration Calculations Complete Lesson vrogue.co Titration Mass Formula [7] c a = (c t × v t × m)/v a. C a is the analyte concentration in molarity; Provide pupils with a few different worked examples of the same. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence. Titration Mass Formula.
From www.ck12.org
Titration (Calculations) Example 3 ( Video ) Chemistry CK12 Titration Mass Formula Provide pupils with a few different worked examples of the same. The titration formula is used to determine the concentration of a particular substance in a solution. C t is the titrant. The concentration of analyte may then be calculated using the following formula: Ma is the molarity of the acid, while mb is the molarity of the base. Va. Titration Mass Formula.
From mavink.com
Titration Labeled Titration Mass Formula C a is the analyte concentration in molarity; The titration formula is used to determine the concentration of a particular substance in a solution. If the titrant and analyte have a 1:1 mole ratio, the formula is molarity (m) of the acid x volume (v) of the acid = molarity (m) of the base x volume (v) of. Provide pupils. Titration Mass Formula.
From www.showme.com
Titration calculations Science, Chemistry, Chemicalreactions Titration Mass Formula Va and vb are the volumes of the acid and base, respectively. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. If the titrant and analyte have a 1:1 mole ratio, the formula is molarity (m) of the. Titration Mass Formula.
From www.youtube.com
Titration Calculations YouTube Titration Mass Formula C t is the titrant. The titration formula is used to determine the concentration of a particular substance in a solution. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. [7] c a = (c t × v. Titration Mass Formula.
From www.youtube.com
Basic Titrations Chemistry Tutorial YouTube Titration Mass Formula In all cases, the reaction chosen for the analysis must. The titration formula is used to determine the concentration of a particular substance in a solution. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. If the titrant. Titration Mass Formula.
From www.ck12.org
Titration (Calculations) Overview ( Video ) Chemistry CK12 Titration Mass Formula The titration formula is used to determine the concentration of a particular substance in a solution. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. If the titrant and analyte have a 1:1 mole ratio, the formula is. Titration Mass Formula.
From www.slideserve.com
PPT Unit 19 Acid Base Equilibria Titrations PowerPoint Presentation Titration Mass Formula Provide pupils with a few different worked examples of the same. If the titrant and analyte have a 1:1 mole ratio, the formula is molarity (m) of the acid x volume (v) of the acid = molarity (m) of the base x volume (v) of. C a is the analyte concentration in molarity; A titration is a volumetric technique in. Titration Mass Formula.
From www.youtube.com
Stoichiometry Problem Titration Calculation YouTube Titration Mass Formula [7] c a = (c t × v t × m)/v a. Provide pupils with a few different worked examples of the same. Va and vb are the volumes of the acid and base, respectively. The mass of a mole of a substance is its relative mass expressed in grams. In all cases, the reaction chosen for the analysis must.. Titration Mass Formula.
From www.slideserve.com
PPT Neutralization & Titration PowerPoint Presentation, free download Titration Mass Formula [7] c a = (c t × v t × m)/v a. The titration formula is used to determine the concentration of a particular substance in a solution. In all cases, the reaction chosen for the analysis must. If the titrant and analyte have a 1:1 mole ratio, the formula is molarity (m) of the acid x volume (v) of. Titration Mass Formula.
From noel-kmclean.blogspot.com
Calculate Molarity of Acid in Titration Titration Mass Formula The volumes of acids and alkali solutions that react with each other can be measured by. In all cases, the reaction chosen for the analysis must. Ma is the molarity of the acid, while mb is the molarity of the base. C a is the analyte concentration in molarity; [7] c a = (c t × v t × m)/v. Titration Mass Formula.
From www.youtube.com
Titration calculation example Chemistry Khan Academy YouTube Titration Mass Formula Va and vb are the volumes of the acid and base, respectively. C t is the titrant. Ma is the molarity of the acid, while mb is the molarity of the base. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. Titration Mass Formula.
From www.chegg.com
Solved PHASE 6 Titration Complete the following steps N Titration Mass Formula The titration formula is used to determine the concentration of a particular substance in a solution. Va and vb are the volumes of the acid and base, respectively. If the titrant and analyte have a 1:1 mole ratio, the formula is molarity (m) of the acid x volume (v) of the acid = molarity (m) of the base x volume. Titration Mass Formula.
From www.youtube.com
GCSE Chemistry Titration calculations worked examples YouTube Titration Mass Formula If the titrant and analyte have a 1:1 mole ratio, the formula is molarity (m) of the acid x volume (v) of the acid = molarity (m) of the base x volume (v) of. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte). Titration Mass Formula.
From www.slideserve.com
PPT TITRATION PowerPoint Presentation, free download ID1459481 Titration Mass Formula In all cases, the reaction chosen for the analysis must. Provide pupils with a few different worked examples of the same. The volumes of acids and alkali solutions that react with each other can be measured by. C a is the analyte concentration in molarity; Ma is the molarity of the acid, while mb is the molarity of the base.. Titration Mass Formula.