What Does The Boiling Point Of A Liquid Depend Upon . The simple definition of boiling point is that it is the temperature at which a liquid boils. For example, the boiling point of water at sea level is 100 °c or 212 °f. The normal boiling point is the temperature at which the vapour pressure is. The boiling point of a liquid is the temperature at which the liquid’s vapour pressure becomes equal to the surrounding atmosphere’s pressure. Boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure. Learn how boiling point depends on pressure and how it is used for liquid purification and cooking. The boiling point of a liquid depends on pressure, vapour pressure and molecular weight. The factors affecting boiling point are. The boiling point of a liquid varies according to the applied pressure; The boiling point is the temperature at which boiling occurs for a specific liquid.
from www.learnatnoon.com
The boiling point of a liquid depends on pressure, vapour pressure and molecular weight. Learn how boiling point depends on pressure and how it is used for liquid purification and cooking. The boiling point of a liquid varies according to the applied pressure; Boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure. The boiling point of a liquid is the temperature at which the liquid’s vapour pressure becomes equal to the surrounding atmosphere’s pressure. The factors affecting boiling point are. The boiling point is the temperature at which boiling occurs for a specific liquid. For example, the boiling point of water at sea level is 100 °c or 212 °f. The simple definition of boiling point is that it is the temperature at which a liquid boils. The normal boiling point is the temperature at which the vapour pressure is.
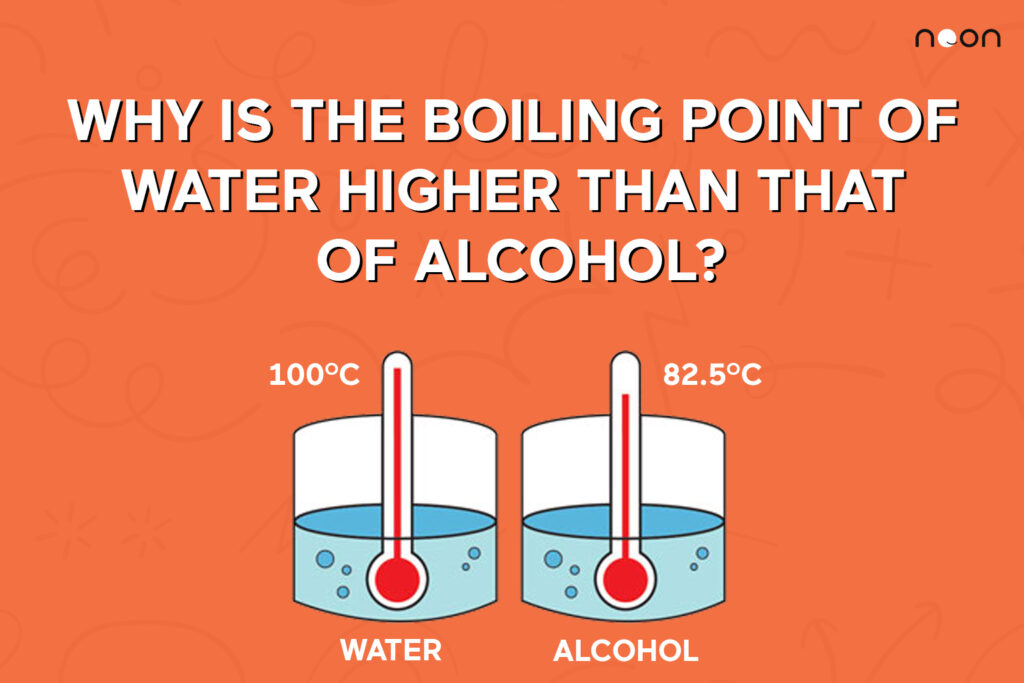
The boiling point of water and alcohol explained Noon Academy
What Does The Boiling Point Of A Liquid Depend Upon Boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure. The boiling point of a liquid varies according to the applied pressure; The boiling point of a liquid depends on pressure, vapour pressure and molecular weight. Learn how boiling point depends on pressure and how it is used for liquid purification and cooking. The factors affecting boiling point are. The simple definition of boiling point is that it is the temperature at which a liquid boils. The boiling point is the temperature at which boiling occurs for a specific liquid. Boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure. The normal boiling point is the temperature at which the vapour pressure is. The boiling point of a liquid is the temperature at which the liquid’s vapour pressure becomes equal to the surrounding atmosphere’s pressure. For example, the boiling point of water at sea level is 100 °c or 212 °f.
From apollo.lsc.vsc.edu
Saturation Vapor Pressure and the Boiling Point What Does The Boiling Point Of A Liquid Depend Upon Boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure. The boiling point is the temperature at which boiling occurs for a specific liquid. The simple definition of boiling point is that it is the temperature at which a liquid boils. Learn how boiling point depends on pressure and how it is used. What Does The Boiling Point Of A Liquid Depend Upon.
From gamesmartz.com
Boiling Point Definition & Image GameSmartz What Does The Boiling Point Of A Liquid Depend Upon For example, the boiling point of water at sea level is 100 °c or 212 °f. The normal boiling point is the temperature at which the vapour pressure is. The boiling point of a liquid is the temperature at which the liquid’s vapour pressure becomes equal to the surrounding atmosphere’s pressure. Boiling point is the temperature at which the vapor. What Does The Boiling Point Of A Liquid Depend Upon.
From www.thoughtco.com
Normal Boiling Point Definition (Chemistry) What Does The Boiling Point Of A Liquid Depend Upon The boiling point of a liquid is the temperature at which the liquid’s vapour pressure becomes equal to the surrounding atmosphere’s pressure. The simple definition of boiling point is that it is the temperature at which a liquid boils. The boiling point of a liquid depends on pressure, vapour pressure and molecular weight. The normal boiling point is the temperature. What Does The Boiling Point Of A Liquid Depend Upon.
From www.youtube.com
Method To Determine Boiling Point Of A Liquid Basic Principles and What Does The Boiling Point Of A Liquid Depend Upon For example, the boiling point of water at sea level is 100 °c or 212 °f. The boiling point of a liquid is the temperature at which the liquid’s vapour pressure becomes equal to the surrounding atmosphere’s pressure. The boiling point of a liquid varies according to the applied pressure; The simple definition of boiling point is that it is. What Does The Boiling Point Of A Liquid Depend Upon.
From engineeringstuff.co.in
What is Boiling point Engineeringstuff What Does The Boiling Point Of A Liquid Depend Upon The boiling point of a liquid is the temperature at which the liquid’s vapour pressure becomes equal to the surrounding atmosphere’s pressure. The factors affecting boiling point are. The normal boiling point is the temperature at which the vapour pressure is. Boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure. The simple. What Does The Boiling Point Of A Liquid Depend Upon.
From www.learnatnoon.com
The boiling point of water and alcohol explained Noon Academy What Does The Boiling Point Of A Liquid Depend Upon Learn how boiling point depends on pressure and how it is used for liquid purification and cooking. The factors affecting boiling point are. The boiling point is the temperature at which boiling occurs for a specific liquid. For example, the boiling point of water at sea level is 100 °c or 212 °f. The boiling point of a liquid depends. What Does The Boiling Point Of A Liquid Depend Upon.
From www.slideserve.com
PPT Ch. 13 States of Matter PowerPoint Presentation, free download What Does The Boiling Point Of A Liquid Depend Upon The simple definition of boiling point is that it is the temperature at which a liquid boils. Boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure. The boiling point is the temperature at which boiling occurs for a specific liquid. The boiling point of a liquid depends on pressure, vapour pressure and. What Does The Boiling Point Of A Liquid Depend Upon.
From spikaph.blogspot.com
Boiling Point Of Water Boiling point of water YouTube For What Does The Boiling Point Of A Liquid Depend Upon The simple definition of boiling point is that it is the temperature at which a liquid boils. The boiling point of a liquid depends on pressure, vapour pressure and molecular weight. The boiling point of a liquid varies according to the applied pressure; The boiling point of a liquid is the temperature at which the liquid’s vapour pressure becomes equal. What Does The Boiling Point Of A Liquid Depend Upon.
From www.healthbenefitstimes.com
Boiling Point Definition of Boiling Point What Does The Boiling Point Of A Liquid Depend Upon The boiling point of a liquid depends on pressure, vapour pressure and molecular weight. The simple definition of boiling point is that it is the temperature at which a liquid boils. For example, the boiling point of water at sea level is 100 °c or 212 °f. The boiling point of a liquid is the temperature at which the liquid’s. What Does The Boiling Point Of A Liquid Depend Upon.
From sciencenotes.org
Boiling Point of Water What Temperature Does Water Boil? What Does The Boiling Point Of A Liquid Depend Upon The boiling point of a liquid is the temperature at which the liquid’s vapour pressure becomes equal to the surrounding atmosphere’s pressure. For example, the boiling point of water at sea level is 100 °c or 212 °f. The simple definition of boiling point is that it is the temperature at which a liquid boils. Learn how boiling point depends. What Does The Boiling Point Of A Liquid Depend Upon.
From www.youtube.com
Evaporation, Vapor Pressure and Boiling YouTube What Does The Boiling Point Of A Liquid Depend Upon The boiling point of a liquid varies according to the applied pressure; The boiling point is the temperature at which boiling occurs for a specific liquid. The normal boiling point is the temperature at which the vapour pressure is. The simple definition of boiling point is that it is the temperature at which a liquid boils. The boiling point of. What Does The Boiling Point Of A Liquid Depend Upon.
From www.youtube.com
Boiling Point of Organic Compounds YouTube What Does The Boiling Point Of A Liquid Depend Upon The boiling point of a liquid is the temperature at which the liquid’s vapour pressure becomes equal to the surrounding atmosphere’s pressure. The boiling point of a liquid varies according to the applied pressure; The normal boiling point is the temperature at which the vapour pressure is. Boiling point is the temperature at which the vapor pressure of a liquid. What Does The Boiling Point Of A Liquid Depend Upon.
From www.slideserve.com
PPT Chapter 10 States of Matter PowerPoint Presentation, free What Does The Boiling Point Of A Liquid Depend Upon For example, the boiling point of water at sea level is 100 °c or 212 °f. Learn how boiling point depends on pressure and how it is used for liquid purification and cooking. Boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure. The simple definition of boiling point is that it is. What Does The Boiling Point Of A Liquid Depend Upon.
From johnfersewing.blogspot.com
What Two Factors Affect the Boiling Point of Water What Does The Boiling Point Of A Liquid Depend Upon The boiling point is the temperature at which boiling occurs for a specific liquid. The normal boiling point is the temperature at which the vapour pressure is. The boiling point of a liquid varies according to the applied pressure; The simple definition of boiling point is that it is the temperature at which a liquid boils. The boiling point of. What Does The Boiling Point Of A Liquid Depend Upon.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps What Does The Boiling Point Of A Liquid Depend Upon The boiling point is the temperature at which boiling occurs for a specific liquid. The boiling point of a liquid is the temperature at which the liquid’s vapour pressure becomes equal to the surrounding atmosphere’s pressure. The factors affecting boiling point are. Boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure. The. What Does The Boiling Point Of A Liquid Depend Upon.
From chemistnotes.com
Boiling Point Definition, Boiling point as physical properties What Does The Boiling Point Of A Liquid Depend Upon The boiling point is the temperature at which boiling occurs for a specific liquid. Learn how boiling point depends on pressure and how it is used for liquid purification and cooking. For example, the boiling point of water at sea level is 100 °c or 212 °f. Boiling point is the temperature at which the vapor pressure of a liquid. What Does The Boiling Point Of A Liquid Depend Upon.
From vdocuments.mx
States, Boiling Point, Melting Point, and Solubility · Boiling Point What Does The Boiling Point Of A Liquid Depend Upon The factors affecting boiling point are. The simple definition of boiling point is that it is the temperature at which a liquid boils. The normal boiling point is the temperature at which the vapour pressure is. Boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure. The boiling point of a liquid is. What Does The Boiling Point Of A Liquid Depend Upon.
From collin-owncreator.blogspot.com
The Boiling Point of a Given Liquid Varies What Does The Boiling Point Of A Liquid Depend Upon The simple definition of boiling point is that it is the temperature at which a liquid boils. Boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure. The boiling point of a liquid is the temperature at which the liquid’s vapour pressure becomes equal to the surrounding atmosphere’s pressure. The boiling point of. What Does The Boiling Point Of A Liquid Depend Upon.
From chemistryskills.com
Definition and Explanation of Boiling Point Chemistry Skills What Does The Boiling Point Of A Liquid Depend Upon The boiling point of a liquid is the temperature at which the liquid’s vapour pressure becomes equal to the surrounding atmosphere’s pressure. The simple definition of boiling point is that it is the temperature at which a liquid boils. Learn how boiling point depends on pressure and how it is used for liquid purification and cooking. Boiling point is the. What Does The Boiling Point Of A Liquid Depend Upon.
From matmake.com
Boiling Point Definition, Measurements, and Applications What Does The Boiling Point Of A Liquid Depend Upon For example, the boiling point of water at sea level is 100 °c or 212 °f. The boiling point of a liquid depends on pressure, vapour pressure and molecular weight. The factors affecting boiling point are. The normal boiling point is the temperature at which the vapour pressure is. The simple definition of boiling point is that it is the. What Does The Boiling Point Of A Liquid Depend Upon.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation ID705859 What Does The Boiling Point Of A Liquid Depend Upon For example, the boiling point of water at sea level is 100 °c or 212 °f. Learn how boiling point depends on pressure and how it is used for liquid purification and cooking. The boiling point is the temperature at which boiling occurs for a specific liquid. The factors affecting boiling point are. Boiling point is the temperature at which. What Does The Boiling Point Of A Liquid Depend Upon.
From www.youtube.com
Boiling point and external pressure YouTube What Does The Boiling Point Of A Liquid Depend Upon The boiling point of a liquid varies according to the applied pressure; For example, the boiling point of water at sea level is 100 °c or 212 °f. The boiling point of a liquid depends on pressure, vapour pressure and molecular weight. Learn how boiling point depends on pressure and how it is used for liquid purification and cooking. The. What Does The Boiling Point Of A Liquid Depend Upon.
From www.vedantu.com
Boiling Point Elevation Learn Important Terms and Concepts What Does The Boiling Point Of A Liquid Depend Upon The factors affecting boiling point are. Learn how boiling point depends on pressure and how it is used for liquid purification and cooking. For example, the boiling point of water at sea level is 100 °c or 212 °f. The boiling point of a liquid varies according to the applied pressure; The normal boiling point is the temperature at which. What Does The Boiling Point Of A Liquid Depend Upon.
From www.youtube.com
Binary Boiling Point Diagram of a LiquidLiquid Mixture YouTube What Does The Boiling Point Of A Liquid Depend Upon The boiling point of a liquid is the temperature at which the liquid’s vapour pressure becomes equal to the surrounding atmosphere’s pressure. The normal boiling point is the temperature at which the vapour pressure is. The boiling point is the temperature at which boiling occurs for a specific liquid. Learn how boiling point depends on pressure and how it is. What Does The Boiling Point Of A Liquid Depend Upon.
From studiousguy.com
Boiling Point Examples in Everyday Life StudiousGuy What Does The Boiling Point Of A Liquid Depend Upon The simple definition of boiling point is that it is the temperature at which a liquid boils. The factors affecting boiling point are. The boiling point is the temperature at which boiling occurs for a specific liquid. The boiling point of a liquid is the temperature at which the liquid’s vapour pressure becomes equal to the surrounding atmosphere’s pressure. The. What Does The Boiling Point Of A Liquid Depend Upon.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps What Does The Boiling Point Of A Liquid Depend Upon The boiling point of a liquid depends on pressure, vapour pressure and molecular weight. The simple definition of boiling point is that it is the temperature at which a liquid boils. The factors affecting boiling point are. The boiling point of a liquid is the temperature at which the liquid’s vapour pressure becomes equal to the surrounding atmosphere’s pressure. For. What Does The Boiling Point Of A Liquid Depend Upon.
From www.youtube.com
What is boiling point what is the boiling point Factors affecting What Does The Boiling Point Of A Liquid Depend Upon Learn how boiling point depends on pressure and how it is used for liquid purification and cooking. The boiling point of a liquid is the temperature at which the liquid’s vapour pressure becomes equal to the surrounding atmosphere’s pressure. The boiling point of a liquid depends on pressure, vapour pressure and molecular weight. The boiling point is the temperature at. What Does The Boiling Point Of A Liquid Depend Upon.
From stock.adobe.com
Boiling and Evaporation, Freezing and Melting Points of Water. Stock What Does The Boiling Point Of A Liquid Depend Upon The boiling point of a liquid varies according to the applied pressure; For example, the boiling point of water at sea level is 100 °c or 212 °f. Boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure. The boiling point of a liquid is the temperature at which the liquid’s vapour pressure. What Does The Boiling Point Of A Liquid Depend Upon.
From www.physicsfox.org
Melting & Boiling • Matter • Physics Fox What Does The Boiling Point Of A Liquid Depend Upon The boiling point is the temperature at which boiling occurs for a specific liquid. The boiling point of a liquid depends on pressure, vapour pressure and molecular weight. For example, the boiling point of water at sea level is 100 °c or 212 °f. The simple definition of boiling point is that it is the temperature at which a liquid. What Does The Boiling Point Of A Liquid Depend Upon.
From www.slideserve.com
PPT Chapter 13 continued… PowerPoint Presentation, free download ID What Does The Boiling Point Of A Liquid Depend Upon The boiling point is the temperature at which boiling occurs for a specific liquid. For example, the boiling point of water at sea level is 100 °c or 212 °f. The boiling point of a liquid is the temperature at which the liquid’s vapour pressure becomes equal to the surrounding atmosphere’s pressure. The boiling point of a liquid varies according. What Does The Boiling Point Of A Liquid Depend Upon.
From www.youtube.com
Melting and Boiling Points p98 (Foundation p97) YouTube What Does The Boiling Point Of A Liquid Depend Upon Boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure. For example, the boiling point of water at sea level is 100 °c or 212 °f. The boiling point of a liquid is the temperature at which the liquid’s vapour pressure becomes equal to the surrounding atmosphere’s pressure. The factors affecting boiling point. What Does The Boiling Point Of A Liquid Depend Upon.
From www.youtube.com
Determine the Boiling Point of a Given Organic Liquid, Chemistry What Does The Boiling Point Of A Liquid Depend Upon The normal boiling point is the temperature at which the vapour pressure is. The boiling point of a liquid is the temperature at which the liquid’s vapour pressure becomes equal to the surrounding atmosphere’s pressure. Boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure. The simple definition of boiling point is that. What Does The Boiling Point Of A Liquid Depend Upon.
From chem.libretexts.org
10.4 Properties of Liquids Chemistry LibreTexts What Does The Boiling Point Of A Liquid Depend Upon Learn how boiling point depends on pressure and how it is used for liquid purification and cooking. The boiling point is the temperature at which boiling occurs for a specific liquid. The normal boiling point is the temperature at which the vapour pressure is. The boiling point of a liquid depends on pressure, vapour pressure and molecular weight. The boiling. What Does The Boiling Point Of A Liquid Depend Upon.
From www.worksheetsplanet.com
What is Boiling Point What Does The Boiling Point Of A Liquid Depend Upon For example, the boiling point of water at sea level is 100 °c or 212 °f. The boiling point of a liquid depends on pressure, vapour pressure and molecular weight. The boiling point of a liquid varies according to the applied pressure; Learn how boiling point depends on pressure and how it is used for liquid purification and cooking. Boiling. What Does The Boiling Point Of A Liquid Depend Upon.
From sciencenotes.org
Boiling Point Definition, Temperature, and Examples What Does The Boiling Point Of A Liquid Depend Upon The boiling point is the temperature at which boiling occurs for a specific liquid. The normal boiling point is the temperature at which the vapour pressure is. For example, the boiling point of water at sea level is 100 °c or 212 °f. Learn how boiling point depends on pressure and how it is used for liquid purification and cooking.. What Does The Boiling Point Of A Liquid Depend Upon.