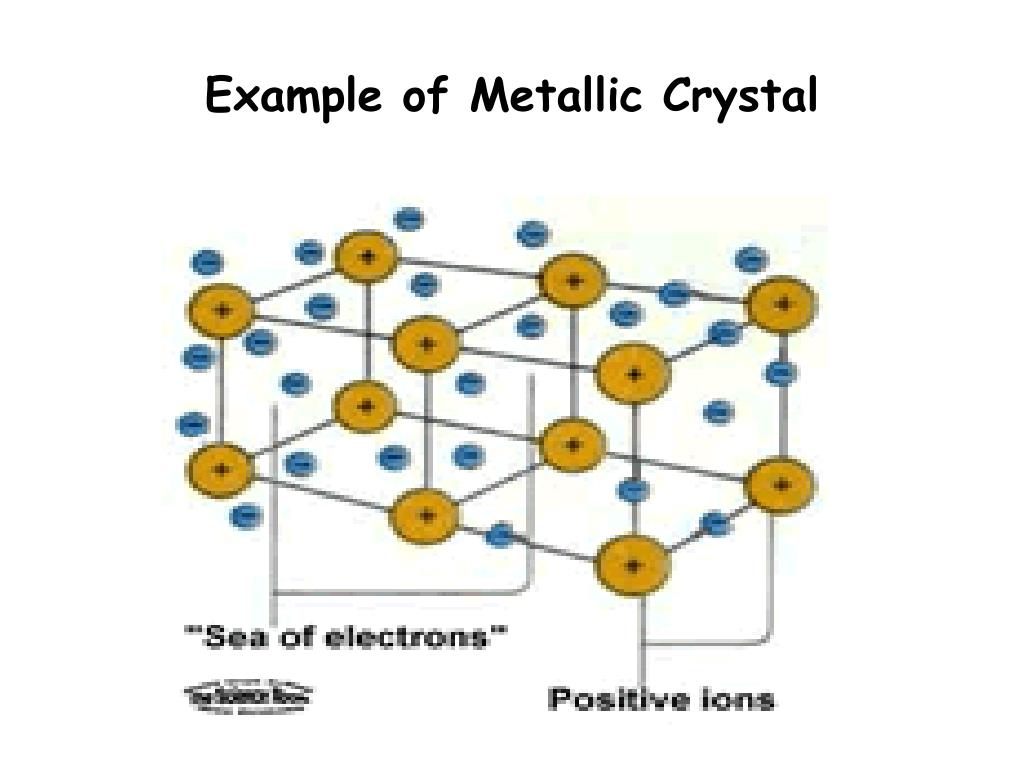
Metallic Crystals Definition And Example . metallic crystals are solid structures formed by metal atoms that are held together by metallic bonds, characterized by a sea of. Metals often form metallic crystals, where some of the valence. metallic crystals are a type of solid structure formed by metal atoms arranged in a regular, repeating pattern. atoms in metals lose electrons to form cations. the structure of metallic crystals is often described as a uniform distribution of atomic nuclei within a “sea”. Metallic bonds (electrostatic interactions between the. Delocalized electrons surround the ions. from rainbow bismuth to sparkling silver, different examples of metallic crystals are an integral part of.
from www.slideserve.com
metallic crystals are a type of solid structure formed by metal atoms arranged in a regular, repeating pattern. Metals often form metallic crystals, where some of the valence. Delocalized electrons surround the ions. atoms in metals lose electrons to form cations. from rainbow bismuth to sparkling silver, different examples of metallic crystals are an integral part of. Metallic bonds (electrostatic interactions between the. the structure of metallic crystals is often described as a uniform distribution of atomic nuclei within a “sea”. metallic crystals are solid structures formed by metal atoms that are held together by metallic bonds, characterized by a sea of.
PPT Objectives 12 Introduction to Chemical Bonding PowerPoint
Metallic Crystals Definition And Example Metallic bonds (electrostatic interactions between the. Delocalized electrons surround the ions. atoms in metals lose electrons to form cations. Metallic bonds (electrostatic interactions between the. metallic crystals are solid structures formed by metal atoms that are held together by metallic bonds, characterized by a sea of. metallic crystals are a type of solid structure formed by metal atoms arranged in a regular, repeating pattern. Metals often form metallic crystals, where some of the valence. the structure of metallic crystals is often described as a uniform distribution of atomic nuclei within a “sea”. from rainbow bismuth to sparkling silver, different examples of metallic crystals are an integral part of.
From www.irocks.com
Splendent, Silvery Arsenopyrite Crystal Mass iRocks Fine Minerals Metallic Crystals Definition And Example metallic crystals are a type of solid structure formed by metal atoms arranged in a regular, repeating pattern. Metals often form metallic crystals, where some of the valence. Delocalized electrons surround the ions. Metallic bonds (electrostatic interactions between the. from rainbow bismuth to sparkling silver, different examples of metallic crystals are an integral part of. the structure. Metallic Crystals Definition And Example.
From www.asianscientist.com
In Pursuit Of Perfect Metal Crystals Asian Scientist Magazine Metallic Crystals Definition And Example Metals often form metallic crystals, where some of the valence. the structure of metallic crystals is often described as a uniform distribution of atomic nuclei within a “sea”. metallic crystals are solid structures formed by metal atoms that are held together by metallic bonds, characterized by a sea of. from rainbow bismuth to sparkling silver, different examples. Metallic Crystals Definition And Example.
From www.slideserve.com
PPT CHAPTER 3 PowerPoint Presentation, free download ID472642 Metallic Crystals Definition And Example Metallic bonds (electrostatic interactions between the. metallic crystals are solid structures formed by metal atoms that are held together by metallic bonds, characterized by a sea of. atoms in metals lose electrons to form cations. metallic crystals are a type of solid structure formed by metal atoms arranged in a regular, repeating pattern. from rainbow bismuth. Metallic Crystals Definition And Example.
From www.slideserve.com
PPT Objectives 12 Introduction to Chemical Bonding PowerPoint Metallic Crystals Definition And Example metallic crystals are a type of solid structure formed by metal atoms arranged in a regular, repeating pattern. Metals often form metallic crystals, where some of the valence. Metallic bonds (electrostatic interactions between the. from rainbow bismuth to sparkling silver, different examples of metallic crystals are an integral part of. atoms in metals lose electrons to form. Metallic Crystals Definition And Example.
From opengeology.org
Earth Materials Mineral Identification Historical Geology Metallic Crystals Definition And Example Metals often form metallic crystals, where some of the valence. metallic crystals are solid structures formed by metal atoms that are held together by metallic bonds, characterized by a sea of. from rainbow bismuth to sparkling silver, different examples of metallic crystals are an integral part of. atoms in metals lose electrons to form cations. Delocalized electrons. Metallic Crystals Definition And Example.
From www.slideserve.com
PPT Metals PowerPoint Presentation, free download ID1128267 Metallic Crystals Definition And Example from rainbow bismuth to sparkling silver, different examples of metallic crystals are an integral part of. atoms in metals lose electrons to form cations. the structure of metallic crystals is often described as a uniform distribution of atomic nuclei within a “sea”. Metals often form metallic crystals, where some of the valence. Metallic bonds (electrostatic interactions between. Metallic Crystals Definition And Example.
From www.britannica.com
Bodycentred cubic structure crystalline form Britannica Metallic Crystals Definition And Example the structure of metallic crystals is often described as a uniform distribution of atomic nuclei within a “sea”. metallic crystals are a type of solid structure formed by metal atoms arranged in a regular, repeating pattern. atoms in metals lose electrons to form cations. Metallic bonds (electrostatic interactions between the. metallic crystals are solid structures formed. Metallic Crystals Definition And Example.
From www.findlight.net
XRay Diffraction Getting to Know Crystal Structures (Part Ⅰ) Metallic Crystals Definition And Example metallic crystals are solid structures formed by metal atoms that are held together by metallic bonds, characterized by a sea of. the structure of metallic crystals is often described as a uniform distribution of atomic nuclei within a “sea”. atoms in metals lose electrons to form cations. Metals often form metallic crystals, where some of the valence.. Metallic Crystals Definition And Example.
From www.thoughtco.com
Metal Crystals Photo Gallery Metallic Crystals Definition And Example atoms in metals lose electrons to form cations. Metals often form metallic crystals, where some of the valence. Delocalized electrons surround the ions. metallic crystals are solid structures formed by metal atoms that are held together by metallic bonds, characterized by a sea of. Metallic bonds (electrostatic interactions between the. metallic crystals are a type of solid. Metallic Crystals Definition And Example.
From www.slideserve.com
PPT Structures of Simple Solids PowerPoint Presentation, free Metallic Crystals Definition And Example metallic crystals are solid structures formed by metal atoms that are held together by metallic bonds, characterized by a sea of. Metallic bonds (electrostatic interactions between the. atoms in metals lose electrons to form cations. from rainbow bismuth to sparkling silver, different examples of metallic crystals are an integral part of. Delocalized electrons surround the ions. . Metallic Crystals Definition And Example.
From www.slideserve.com
PPT Properties of Minerals PowerPoint Presentation, free download Metallic Crystals Definition And Example metallic crystals are solid structures formed by metal atoms that are held together by metallic bonds, characterized by a sea of. Metals often form metallic crystals, where some of the valence. Delocalized electrons surround the ions. Metallic bonds (electrostatic interactions between the. atoms in metals lose electrons to form cations. the structure of metallic crystals is often. Metallic Crystals Definition And Example.
From sciencenotes.org
How to Grow Metal Crystals Metallic Crystals Definition And Example metallic crystals are a type of solid structure formed by metal atoms arranged in a regular, repeating pattern. the structure of metallic crystals is often described as a uniform distribution of atomic nuclei within a “sea”. Delocalized electrons surround the ions. from rainbow bismuth to sparkling silver, different examples of metallic crystals are an integral part of.. Metallic Crystals Definition And Example.
From opengeology.org
13 Crystal Structures Mineralogy Metallic Crystals Definition And Example Metallic bonds (electrostatic interactions between the. Metals often form metallic crystals, where some of the valence. atoms in metals lose electrons to form cations. metallic crystals are solid structures formed by metal atoms that are held together by metallic bonds, characterized by a sea of. the structure of metallic crystals is often described as a uniform distribution. Metallic Crystals Definition And Example.
From www.slideserve.com
PPT Structures of Simple Solids PowerPoint Presentation, free Metallic Crystals Definition And Example metallic crystals are solid structures formed by metal atoms that are held together by metallic bonds, characterized by a sea of. from rainbow bismuth to sparkling silver, different examples of metallic crystals are an integral part of. atoms in metals lose electrons to form cations. Metallic bonds (electrostatic interactions between the. the structure of metallic crystals. Metallic Crystals Definition And Example.
From www.schoolchalao.com
Metallic Minerals Metallic Crystals Definition And Example atoms in metals lose electrons to form cations. Metallic bonds (electrostatic interactions between the. from rainbow bismuth to sparkling silver, different examples of metallic crystals are an integral part of. metallic crystals are solid structures formed by metal atoms that are held together by metallic bonds, characterized by a sea of. Metals often form metallic crystals, where. Metallic Crystals Definition And Example.
From chem.libretexts.org
12.6 Crystal Structures Chemistry LibreTexts Metallic Crystals Definition And Example from rainbow bismuth to sparkling silver, different examples of metallic crystals are an integral part of. the structure of metallic crystals is often described as a uniform distribution of atomic nuclei within a “sea”. metallic crystals are solid structures formed by metal atoms that are held together by metallic bonds, characterized by a sea of. atoms. Metallic Crystals Definition And Example.
From www.slideserve.com
PPT Chemistry Bonding Types of Bonds PowerPoint Presentation, free Metallic Crystals Definition And Example Metallic bonds (electrostatic interactions between the. atoms in metals lose electrons to form cations. the structure of metallic crystals is often described as a uniform distribution of atomic nuclei within a “sea”. from rainbow bismuth to sparkling silver, different examples of metallic crystals are an integral part of. Delocalized electrons surround the ions. metallic crystals are. Metallic Crystals Definition And Example.
From www.britannica.com
Metallic bond Properties, Examples, & Explanation Britannica Metallic Crystals Definition And Example from rainbow bismuth to sparkling silver, different examples of metallic crystals are an integral part of. Delocalized electrons surround the ions. the structure of metallic crystals is often described as a uniform distribution of atomic nuclei within a “sea”. atoms in metals lose electrons to form cations. metallic crystals are solid structures formed by metal atoms. Metallic Crystals Definition And Example.
From www.slideserve.com
PPT Structures of Simple Solids PowerPoint Presentation, free Metallic Crystals Definition And Example atoms in metals lose electrons to form cations. Delocalized electrons surround the ions. from rainbow bismuth to sparkling silver, different examples of metallic crystals are an integral part of. Metals often form metallic crystals, where some of the valence. metallic crystals are solid structures formed by metal atoms that are held together by metallic bonds, characterized by. Metallic Crystals Definition And Example.
From www.thoughtco.com
Metal Crystals Photo Gallery Metallic Crystals Definition And Example from rainbow bismuth to sparkling silver, different examples of metallic crystals are an integral part of. metallic crystals are a type of solid structure formed by metal atoms arranged in a regular, repeating pattern. the structure of metallic crystals is often described as a uniform distribution of atomic nuclei within a “sea”. Metals often form metallic crystals,. Metallic Crystals Definition And Example.
From dxosyxudp.blob.core.windows.net
What Are The Characteristics Of Stones at Emery Lawrence blog Metallic Crystals Definition And Example Metallic bonds (electrostatic interactions between the. metallic crystals are a type of solid structure formed by metal atoms arranged in a regular, repeating pattern. atoms in metals lose electrons to form cations. the structure of metallic crystals is often described as a uniform distribution of atomic nuclei within a “sea”. Metals often form metallic crystals, where some. Metallic Crystals Definition And Example.
From www.slideserve.com
PPT Chapter 3 Structures of Metals & Ceramics PowerPoint Metallic Crystals Definition And Example Delocalized electrons surround the ions. from rainbow bismuth to sparkling silver, different examples of metallic crystals are an integral part of. metallic crystals are solid structures formed by metal atoms that are held together by metallic bonds, characterized by a sea of. metallic crystals are a type of solid structure formed by metal atoms arranged in a. Metallic Crystals Definition And Example.
From thatcrystalsite.com
15 Of The Best Metallic Crystals That Crystal Site Metallic Crystals Definition And Example from rainbow bismuth to sparkling silver, different examples of metallic crystals are an integral part of. the structure of metallic crystals is often described as a uniform distribution of atomic nuclei within a “sea”. metallic crystals are a type of solid structure formed by metal atoms arranged in a regular, repeating pattern. Metallic bonds (electrostatic interactions between. Metallic Crystals Definition And Example.
From www.slideserve.com
PPT Properties of Solids PowerPoint Presentation, free download ID Metallic Crystals Definition And Example Delocalized electrons surround the ions. Metallic bonds (electrostatic interactions between the. the structure of metallic crystals is often described as a uniform distribution of atomic nuclei within a “sea”. from rainbow bismuth to sparkling silver, different examples of metallic crystals are an integral part of. Metals often form metallic crystals, where some of the valence. metallic crystals. Metallic Crystals Definition And Example.
From www.slideserve.com
PPT SOLID STATE CHEMISTRY PowerPoint Presentation, free download ID Metallic Crystals Definition And Example from rainbow bismuth to sparkling silver, different examples of metallic crystals are an integral part of. metallic crystals are solid structures formed by metal atoms that are held together by metallic bonds, characterized by a sea of. atoms in metals lose electrons to form cations. Metallic bonds (electrostatic interactions between the. the structure of metallic crystals. Metallic Crystals Definition And Example.
From www.slideserve.com
PPT CHAPTER 10 STATES OF MATTER PowerPoint Presentation, free Metallic Crystals Definition And Example the structure of metallic crystals is often described as a uniform distribution of atomic nuclei within a “sea”. Metals often form metallic crystals, where some of the valence. Delocalized electrons surround the ions. from rainbow bismuth to sparkling silver, different examples of metallic crystals are an integral part of. metallic crystals are solid structures formed by metal. Metallic Crystals Definition And Example.
From www.slideshare.net
Crystal structure of metal Metallic Crystals Definition And Example atoms in metals lose electrons to form cations. the structure of metallic crystals is often described as a uniform distribution of atomic nuclei within a “sea”. Delocalized electrons surround the ions. metallic crystals are solid structures formed by metal atoms that are held together by metallic bonds, characterized by a sea of. from rainbow bismuth to. Metallic Crystals Definition And Example.
From www.youtube.com
Metallic, Ionic, and Molecular Solids Explained with Metallic Crystals Definition And Example Delocalized electrons surround the ions. from rainbow bismuth to sparkling silver, different examples of metallic crystals are an integral part of. metallic crystals are solid structures formed by metal atoms that are held together by metallic bonds, characterized by a sea of. Metals often form metallic crystals, where some of the valence. metallic crystals are a type. Metallic Crystals Definition And Example.
From www.slideshare.net
Crystal structure of metal Metallic Crystals Definition And Example atoms in metals lose electrons to form cations. from rainbow bismuth to sparkling silver, different examples of metallic crystals are an integral part of. metallic crystals are a type of solid structure formed by metal atoms arranged in a regular, repeating pattern. Metallic bonds (electrostatic interactions between the. the structure of metallic crystals is often described. Metallic Crystals Definition And Example.
From www.youtube.com
Metallic Crystals YouTube Metallic Crystals Definition And Example from rainbow bismuth to sparkling silver, different examples of metallic crystals are an integral part of. Metallic bonds (electrostatic interactions between the. atoms in metals lose electrons to form cations. metallic crystals are a type of solid structure formed by metal atoms arranged in a regular, repeating pattern. Delocalized electrons surround the ions. the structure of. Metallic Crystals Definition And Example.
From www.slideserve.com
PPT Structures of Simple Solids PowerPoint Presentation, free Metallic Crystals Definition And Example Delocalized electrons surround the ions. Metallic bonds (electrostatic interactions between the. metallic crystals are a type of solid structure formed by metal atoms arranged in a regular, repeating pattern. Metals often form metallic crystals, where some of the valence. metallic crystals are solid structures formed by metal atoms that are held together by metallic bonds, characterized by a. Metallic Crystals Definition And Example.
From www.thoughtco.com
Metal Crystals Photo Gallery Metallic Crystals Definition And Example metallic crystals are solid structures formed by metal atoms that are held together by metallic bonds, characterized by a sea of. the structure of metallic crystals is often described as a uniform distribution of atomic nuclei within a “sea”. from rainbow bismuth to sparkling silver, different examples of metallic crystals are an integral part of. Delocalized electrons. Metallic Crystals Definition And Example.
From www.slideserve.com
PPT Solids PowerPoint Presentation, free download ID9479725 Metallic Crystals Definition And Example metallic crystals are solid structures formed by metal atoms that are held together by metallic bonds, characterized by a sea of. Metals often form metallic crystals, where some of the valence. metallic crystals are a type of solid structure formed by metal atoms arranged in a regular, repeating pattern. Delocalized electrons surround the ions. from rainbow bismuth. Metallic Crystals Definition And Example.
From www.slideserve.com
PPT Structures of Simple Solids PowerPoint Presentation, free Metallic Crystals Definition And Example Metals often form metallic crystals, where some of the valence. the structure of metallic crystals is often described as a uniform distribution of atomic nuclei within a “sea”. from rainbow bismuth to sparkling silver, different examples of metallic crystals are an integral part of. Metallic bonds (electrostatic interactions between the. metallic crystals are solid structures formed by. Metallic Crystals Definition And Example.
From www.thoughtco.com
Metal Crystals Photo Gallery Metallic Crystals Definition And Example metallic crystals are solid structures formed by metal atoms that are held together by metallic bonds, characterized by a sea of. Metallic bonds (electrostatic interactions between the. from rainbow bismuth to sparkling silver, different examples of metallic crystals are an integral part of. the structure of metallic crystals is often described as a uniform distribution of atomic. Metallic Crystals Definition And Example.