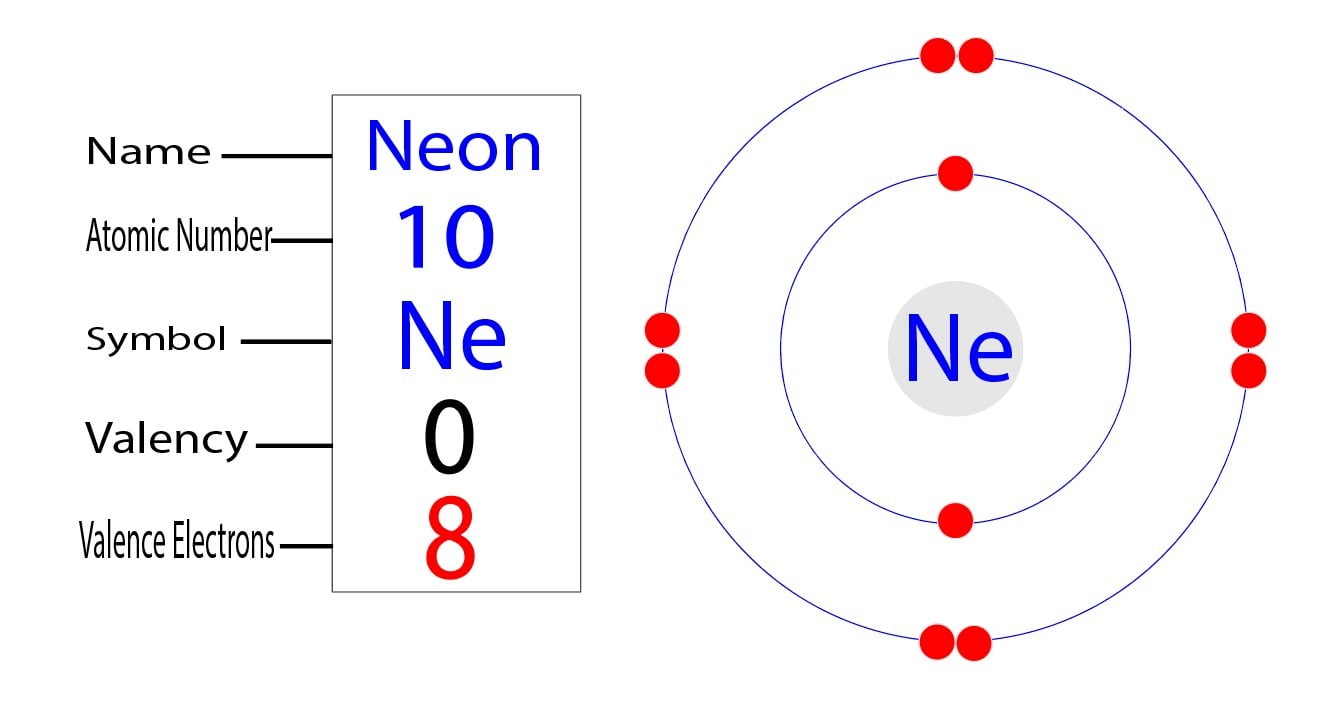
Iron Have Many Electrons . Electrons are negatively charged particles. Iron has 26 electrons, matching the number of protons. The core of the earth is thought to be largely composed of iron with. Iron is the fourth most abundant element, by mass, in the earth’s crust. The ground state electronic configuration of neutral iron is [ar]. Fe, fe2+, and fe3+ electron. Iron is a chemical element with atomic number 26 which means there are 26. When we write the configuration we'll put all 26 electrons in orbitals around the nucleus of the iron atom. They orbit the nucleus in. As a result, iron has eight valence electrons. Iron atoms have 26 electrons and the shell structure is 2.8.14.2.
from valenceelectrons.com
Iron is the fourth most abundant element, by mass, in the earth’s crust. Fe, fe2+, and fe3+ electron. Iron is a chemical element with atomic number 26 which means there are 26. As a result, iron has eight valence electrons. Iron has 26 electrons, matching the number of protons. When we write the configuration we'll put all 26 electrons in orbitals around the nucleus of the iron atom. Electrons are negatively charged particles. The core of the earth is thought to be largely composed of iron with. The ground state electronic configuration of neutral iron is [ar]. They orbit the nucleus in.
How to Find the Valence Electrons for Iron (Fe)?
Iron Have Many Electrons Fe, fe2+, and fe3+ electron. As a result, iron has eight valence electrons. Electrons are negatively charged particles. Iron is a chemical element with atomic number 26 which means there are 26. Iron is the fourth most abundant element, by mass, in the earth’s crust. The ground state electronic configuration of neutral iron is [ar]. Iron has 26 electrons, matching the number of protons. Fe, fe2+, and fe3+ electron. They orbit the nucleus in. The core of the earth is thought to be largely composed of iron with. Iron atoms have 26 electrons and the shell structure is 2.8.14.2. When we write the configuration we'll put all 26 electrons in orbitals around the nucleus of the iron atom.
From howtodiscuss.com
How many Valence Electrons does Iron have? How To Discuss Iron Have Many Electrons Fe, fe2+, and fe3+ electron. Electrons are negatively charged particles. They orbit the nucleus in. The ground state electronic configuration of neutral iron is [ar]. When we write the configuration we'll put all 26 electrons in orbitals around the nucleus of the iron atom. Iron is the fourth most abundant element, by mass, in the earth’s crust. Iron is a. Iron Have Many Electrons.
From www.bigstockphoto.com
Iron. Atom Structure Vector & Photo (Free Trial) Bigstock Iron Have Many Electrons Fe, fe2+, and fe3+ electron. The core of the earth is thought to be largely composed of iron with. The ground state electronic configuration of neutral iron is [ar]. When we write the configuration we'll put all 26 electrons in orbitals around the nucleus of the iron atom. Iron is a chemical element with atomic number 26 which means there. Iron Have Many Electrons.
From cetqppft.blob.core.windows.net
What Is The Characteristic Valence Shell Electron Configuration Of 11Th Iron Have Many Electrons They orbit the nucleus in. As a result, iron has eight valence electrons. Iron atoms have 26 electrons and the shell structure is 2.8.14.2. Iron is a chemical element with atomic number 26 which means there are 26. Electrons are negatively charged particles. The core of the earth is thought to be largely composed of iron with. Iron is the. Iron Have Many Electrons.
From www.youtube.com
How to find Protons & Electrons for Fe2+ and Fe3+ (Iron II and III ions Iron Have Many Electrons Iron is a chemical element with atomic number 26 which means there are 26. The core of the earth is thought to be largely composed of iron with. Electrons are negatively charged particles. They orbit the nucleus in. The ground state electronic configuration of neutral iron is [ar]. Iron is the fourth most abundant element, by mass, in the earth’s. Iron Have Many Electrons.
From valenceelectrons.com
How many protons, neutrons and electrons does iron have? Iron Have Many Electrons They orbit the nucleus in. Iron is the fourth most abundant element, by mass, in the earth’s crust. Iron is a chemical element with atomic number 26 which means there are 26. Iron has 26 electrons, matching the number of protons. The core of the earth is thought to be largely composed of iron with. When we write the configuration. Iron Have Many Electrons.
From valenceelectrons.com
How to Find the Valence Electrons for Iron (Fe)? Iron Have Many Electrons Fe, fe2+, and fe3+ electron. Iron is the fourth most abundant element, by mass, in the earth’s crust. Iron is a chemical element with atomic number 26 which means there are 26. The ground state electronic configuration of neutral iron is [ar]. Electrons are negatively charged particles. Iron has 26 electrons, matching the number of protons. The core of the. Iron Have Many Electrons.
From elements4kids.wordpress.com
26 Iron (Fe) Elements4Kids Iron Have Many Electrons As a result, iron has eight valence electrons. When we write the configuration we'll put all 26 electrons in orbitals around the nucleus of the iron atom. Iron atoms have 26 electrons and the shell structure is 2.8.14.2. They orbit the nucleus in. Iron is the fourth most abundant element, by mass, in the earth’s crust. The core of the. Iron Have Many Electrons.
From www.dreamstime.com
Iron Atom Stock Illustrations 1,568 Iron Atom Stock Illustrations Iron Have Many Electrons Iron has 26 electrons, matching the number of protons. Fe, fe2+, and fe3+ electron. Iron is the fourth most abundant element, by mass, in the earth’s crust. The ground state electronic configuration of neutral iron is [ar]. The core of the earth is thought to be largely composed of iron with. Electrons are negatively charged particles. They orbit the nucleus. Iron Have Many Electrons.
From material-properties.org
Iron Protons Neutrons Electrons Electron Configuration Iron Have Many Electrons Fe, fe2+, and fe3+ electron. Electrons are negatively charged particles. The core of the earth is thought to be largely composed of iron with. Iron atoms have 26 electrons and the shell structure is 2.8.14.2. The ground state electronic configuration of neutral iron is [ar]. Iron is a chemical element with atomic number 26 which means there are 26. Iron. Iron Have Many Electrons.
From reviewhomedecor.co
Iron Periodic Table Electrons Review Home Decor Iron Have Many Electrons When we write the configuration we'll put all 26 electrons in orbitals around the nucleus of the iron atom. Iron is the fourth most abundant element, by mass, in the earth’s crust. Fe, fe2+, and fe3+ electron. The ground state electronic configuration of neutral iron is [ar]. Iron atoms have 26 electrons and the shell structure is 2.8.14.2. They orbit. Iron Have Many Electrons.
From ceaulups.blob.core.windows.net
Iron Has How Many Electrons In The Shells at Nicole Paige blog Iron Have Many Electrons When we write the configuration we'll put all 26 electrons in orbitals around the nucleus of the iron atom. Iron has 26 electrons, matching the number of protons. They orbit the nucleus in. As a result, iron has eight valence electrons. Electrons are negatively charged particles. The ground state electronic configuration of neutral iron is [ar]. Iron is a chemical. Iron Have Many Electrons.
From www.youtube.com
Valence Electrons for Fe (Iron) YouTube Iron Have Many Electrons Fe, fe2+, and fe3+ electron. Iron atoms have 26 electrons and the shell structure is 2.8.14.2. They orbit the nucleus in. Electrons are negatively charged particles. As a result, iron has eight valence electrons. When we write the configuration we'll put all 26 electrons in orbitals around the nucleus of the iron atom. Iron is a chemical element with atomic. Iron Have Many Electrons.
From www.numerade.com
SOLVEDIn iron atom, how many electrons atom have n=3 and l=2 ? (a) 2 Iron Have Many Electrons The core of the earth is thought to be largely composed of iron with. As a result, iron has eight valence electrons. The ground state electronic configuration of neutral iron is [ar]. Electrons are negatively charged particles. When we write the configuration we'll put all 26 electrons in orbitals around the nucleus of the iron atom. Fe, fe2+, and fe3+. Iron Have Many Electrons.
From www.youtube.com
How to find the Number of Protons, Electrons, Neutrons for Iron (Fe Iron Have Many Electrons Iron has 26 electrons, matching the number of protons. The ground state electronic configuration of neutral iron is [ar]. Fe, fe2+, and fe3+ electron. Iron is the fourth most abundant element, by mass, in the earth’s crust. As a result, iron has eight valence electrons. Iron atoms have 26 electrons and the shell structure is 2.8.14.2. When we write the. Iron Have Many Electrons.
From rapidelectron.blogspot.com
Electron Configuration For An Atom Of Iron Rapid Electron Iron Have Many Electrons Iron has 26 electrons, matching the number of protons. The core of the earth is thought to be largely composed of iron with. Iron atoms have 26 electrons and the shell structure is 2.8.14.2. Fe, fe2+, and fe3+ electron. Iron is the fourth most abundant element, by mass, in the earth’s crust. They orbit the nucleus in. Electrons are negatively. Iron Have Many Electrons.
From www.tes.com
2.2 Electronic Structure & the Periodic table Teaching Resources Iron Have Many Electrons Iron atoms have 26 electrons and the shell structure is 2.8.14.2. They orbit the nucleus in. When we write the configuration we'll put all 26 electrons in orbitals around the nucleus of the iron atom. Iron is the fourth most abundant element, by mass, in the earth’s crust. The ground state electronic configuration of neutral iron is [ar]. Electrons are. Iron Have Many Electrons.
From awesomehome.co
Iron Periodic Table Electrons Awesome Home Iron Have Many Electrons Iron has 26 electrons, matching the number of protons. Electrons are negatively charged particles. Iron is a chemical element with atomic number 26 which means there are 26. When we write the configuration we'll put all 26 electrons in orbitals around the nucleus of the iron atom. As a result, iron has eight valence electrons. Fe, fe2+, and fe3+ electron.. Iron Have Many Electrons.
From new--electronic.blogspot.com
Electron Configuration Of Ferrous Ion Iron Have Many Electrons The core of the earth is thought to be largely composed of iron with. Fe, fe2+, and fe3+ electron. Electrons are negatively charged particles. The ground state electronic configuration of neutral iron is [ar]. Iron is a chemical element with atomic number 26 which means there are 26. As a result, iron has eight valence electrons. Iron is the fourth. Iron Have Many Electrons.
From www.youtube.com
How many valence electrons does iron have? YouTube Iron Have Many Electrons Iron is the fourth most abundant element, by mass, in the earth’s crust. Iron atoms have 26 electrons and the shell structure is 2.8.14.2. The core of the earth is thought to be largely composed of iron with. The ground state electronic configuration of neutral iron is [ar]. As a result, iron has eight valence electrons. When we write the. Iron Have Many Electrons.
From valenceelectrons.com
How Many Protons, Neutrons and Electrons Does Iron Have? Iron Have Many Electrons Iron is the fourth most abundant element, by mass, in the earth’s crust. Iron is a chemical element with atomic number 26 which means there are 26. Fe, fe2+, and fe3+ electron. As a result, iron has eight valence electrons. Electrons are negatively charged particles. Iron has 26 electrons, matching the number of protons. They orbit the nucleus in. Iron. Iron Have Many Electrons.
From valenceelectrons.com
How many valence electrons does iron(Fe) have? Iron Have Many Electrons Fe, fe2+, and fe3+ electron. Iron is the fourth most abundant element, by mass, in the earth’s crust. As a result, iron has eight valence electrons. Iron atoms have 26 electrons and the shell structure is 2.8.14.2. Iron has 26 electrons, matching the number of protons. The core of the earth is thought to be largely composed of iron with.. Iron Have Many Electrons.
From ar.inspiredpencil.com
Iron Orbital Notation Iron Have Many Electrons The ground state electronic configuration of neutral iron is [ar]. They orbit the nucleus in. Iron atoms have 26 electrons and the shell structure is 2.8.14.2. Fe, fe2+, and fe3+ electron. As a result, iron has eight valence electrons. Iron is a chemical element with atomic number 26 which means there are 26. Electrons are negatively charged particles. The core. Iron Have Many Electrons.
From sciencenotes.org
Iron Atom Science Notes and Projects Iron Have Many Electrons Electrons are negatively charged particles. Iron is a chemical element with atomic number 26 which means there are 26. As a result, iron has eight valence electrons. They orbit the nucleus in. Iron is the fourth most abundant element, by mass, in the earth’s crust. Fe, fe2+, and fe3+ electron. The core of the earth is thought to be largely. Iron Have Many Electrons.
From ar.inspiredpencil.com
Electron Configuration Of Iron Iron Have Many Electrons Iron is the fourth most abundant element, by mass, in the earth’s crust. The core of the earth is thought to be largely composed of iron with. Iron has 26 electrons, matching the number of protons. Iron is a chemical element with atomic number 26 which means there are 26. When we write the configuration we'll put all 26 electrons. Iron Have Many Electrons.
From elchoroukhost.net
Iron Periodic Table Protons Neutrons And Electrons Elcho Table Iron Have Many Electrons Iron is a chemical element with atomic number 26 which means there are 26. Fe, fe2+, and fe3+ electron. The ground state electronic configuration of neutral iron is [ar]. They orbit the nucleus in. The core of the earth is thought to be largely composed of iron with. As a result, iron has eight valence electrons. Electrons are negatively charged. Iron Have Many Electrons.
From valenceelectrons.com
How Many Valence Electrons Does Iron (Fe) Have? Iron Have Many Electrons Iron is a chemical element with atomic number 26 which means there are 26. Iron has 26 electrons, matching the number of protons. Iron is the fourth most abundant element, by mass, in the earth’s crust. As a result, iron has eight valence electrons. Fe, fe2+, and fe3+ electron. They orbit the nucleus in. Electrons are negatively charged particles. Iron. Iron Have Many Electrons.
From valenceelectrons.com
How Many Protons, Neutrons and Electrons Does Iron Have? Iron Have Many Electrons Iron has 26 electrons, matching the number of protons. Iron is a chemical element with atomic number 26 which means there are 26. When we write the configuration we'll put all 26 electrons in orbitals around the nucleus of the iron atom. Fe, fe2+, and fe3+ electron. Iron atoms have 26 electrons and the shell structure is 2.8.14.2. Electrons are. Iron Have Many Electrons.
From www.sciencephoto.com
Iron, atomic structure Stock Image C018/3707 Science Photo Library Iron Have Many Electrons Fe, fe2+, and fe3+ electron. The core of the earth is thought to be largely composed of iron with. As a result, iron has eight valence electrons. Electrons are negatively charged particles. They orbit the nucleus in. When we write the configuration we'll put all 26 electrons in orbitals around the nucleus of the iron atom. Iron atoms have 26. Iron Have Many Electrons.
From anelementaday.wordpress.com
Day 3 Iron An Element A Day Iron Have Many Electrons They orbit the nucleus in. Iron has 26 electrons, matching the number of protons. Iron is the fourth most abundant element, by mass, in the earth’s crust. The core of the earth is thought to be largely composed of iron with. Iron atoms have 26 electrons and the shell structure is 2.8.14.2. As a result, iron has eight valence electrons.. Iron Have Many Electrons.
From stock.adobe.com
Iron atomic structure has atomic number, atomic mass, electron Iron Have Many Electrons Iron atoms have 26 electrons and the shell structure is 2.8.14.2. The ground state electronic configuration of neutral iron is [ar]. Iron is the fourth most abundant element, by mass, in the earth’s crust. They orbit the nucleus in. Iron has 26 electrons, matching the number of protons. Iron is a chemical element with atomic number 26 which means there. Iron Have Many Electrons.
From pnghut.com
Electron Configuration Atomic Orbital Shell Energy Level Iron Iron Have Many Electrons Iron has 26 electrons, matching the number of protons. The core of the earth is thought to be largely composed of iron with. The ground state electronic configuration of neutral iron is [ar]. When we write the configuration we'll put all 26 electrons in orbitals around the nucleus of the iron atom. Iron atoms have 26 electrons and the shell. Iron Have Many Electrons.
From www.britannica.com
Iron Element, Occurrence, Uses, Properties, & Compounds Britannica Iron Have Many Electrons Iron is a chemical element with atomic number 26 which means there are 26. Iron atoms have 26 electrons and the shell structure is 2.8.14.2. Electrons are negatively charged particles. Iron has 26 electrons, matching the number of protons. They orbit the nucleus in. As a result, iron has eight valence electrons. Iron is the fourth most abundant element, by. Iron Have Many Electrons.
From www.alamy.com
Iron Chemical Element Stock Photos & Iron Chemical Element Stock Images Iron Have Many Electrons Iron atoms have 26 electrons and the shell structure is 2.8.14.2. As a result, iron has eight valence electrons. Electrons are negatively charged particles. Fe, fe2+, and fe3+ electron. The ground state electronic configuration of neutral iron is [ar]. The core of the earth is thought to be largely composed of iron with. They orbit the nucleus in. Iron has. Iron Have Many Electrons.
From chemistry291.blogspot.com
【5 Steps】Electron Configuration of Iron(Fe) Electron configuration Iron Have Many Electrons Iron atoms have 26 electrons and the shell structure is 2.8.14.2. They orbit the nucleus in. Iron has 26 electrons, matching the number of protons. Electrons are negatively charged particles. Iron is a chemical element with atomic number 26 which means there are 26. When we write the configuration we'll put all 26 electrons in orbitals around the nucleus of. Iron Have Many Electrons.
From utedzz.blogspot.com
Periodic Table Iron Valence Electrons Periodic Table Timeline Iron Have Many Electrons Iron has 26 electrons, matching the number of protons. Iron is a chemical element with atomic number 26 which means there are 26. The core of the earth is thought to be largely composed of iron with. Iron is the fourth most abundant element, by mass, in the earth’s crust. They orbit the nucleus in. When we write the configuration. Iron Have Many Electrons.