Electrochemistry Voltage . a voltmeter measures the difference in the electrochemical potential of electrons (μ̅ e), because it is this difference that drives the current. one volt (v) is the potential difference necessary to generate a charge of 1 coulomb (c) from 1 joule (j) of energy. the standard cell potential for a redox reaction (e° cell) is a measure of the tendency of reactants in their standard. For a voltaic cell, this potential difference is called the cell potential (or emf for electromotive force, although it is not really a force), which is denoted ecell. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. the cell potential is the way in which we can measure how much voltage exists between the two half cells of a battery. Electrochemistry is an interfacial science between.
from www.youtube.com
one volt (v) is the potential difference necessary to generate a charge of 1 coulomb (c) from 1 joule (j) of energy. Electrochemistry is an interfacial science between. For a voltaic cell, this potential difference is called the cell potential (or emf for electromotive force, although it is not really a force), which is denoted ecell. a voltmeter measures the difference in the electrochemical potential of electrons (μ̅ e), because it is this difference that drives the current. the standard cell potential for a redox reaction (e° cell) is a measure of the tendency of reactants in their standard. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. the cell potential is the way in which we can measure how much voltage exists between the two half cells of a battery.
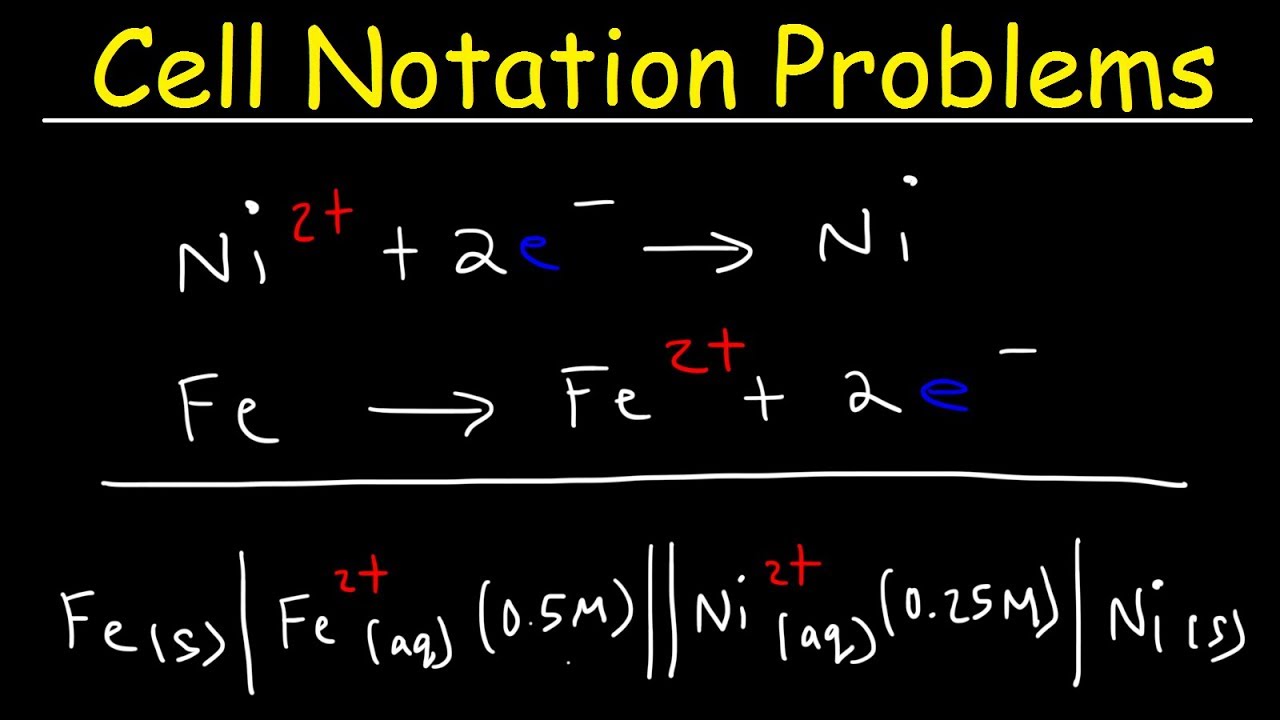
Cell Notation Practice Problems, Voltaic Cells Electrochemistry YouTube
Electrochemistry Voltage a voltmeter measures the difference in the electrochemical potential of electrons (μ̅ e), because it is this difference that drives the current. one volt (v) is the potential difference necessary to generate a charge of 1 coulomb (c) from 1 joule (j) of energy. For a voltaic cell, this potential difference is called the cell potential (or emf for electromotive force, although it is not really a force), which is denoted ecell. the cell potential is the way in which we can measure how much voltage exists between the two half cells of a battery. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. the standard cell potential for a redox reaction (e° cell) is a measure of the tendency of reactants in their standard. a voltmeter measures the difference in the electrochemical potential of electrons (μ̅ e), because it is this difference that drives the current. Electrochemistry is an interfacial science between.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Electrochemistry Voltage For a voltaic cell, this potential difference is called the cell potential (or emf for electromotive force, although it is not really a force), which is denoted ecell. the cell potential is the way in which we can measure how much voltage exists between the two half cells of a battery. the galvanic cell, or called voltaic cell,. Electrochemistry Voltage.
From chemistry.stackexchange.com
electrochemistry Understanding the concept of voltage in electrode Electrochemistry Voltage the standard cell potential for a redox reaction (e° cell) is a measure of the tendency of reactants in their standard. one volt (v) is the potential difference necessary to generate a charge of 1 coulomb (c) from 1 joule (j) of energy. the cell potential is the way in which we can measure how much voltage. Electrochemistry Voltage.
From alevelchemistry.co.uk
Electrochemical Cells Definition, Description & Types Electrochemistry Voltage Electrochemistry is an interfacial science between. the standard cell potential for a redox reaction (e° cell) is a measure of the tendency of reactants in their standard. one volt (v) is the potential difference necessary to generate a charge of 1 coulomb (c) from 1 joule (j) of energy. a voltmeter measures the difference in the electrochemical. Electrochemistry Voltage.
From www.youtube.com
Cell Notation Practice Problems, Voltaic Cells Electrochemistry YouTube Electrochemistry Voltage For a voltaic cell, this potential difference is called the cell potential (or emf for electromotive force, although it is not really a force), which is denoted ecell. one volt (v) is the potential difference necessary to generate a charge of 1 coulomb (c) from 1 joule (j) of energy. Electrochemistry is an interfacial science between. a voltmeter. Electrochemistry Voltage.
From www.researchgate.net
The electrochemical stability window of electrolytes a Linear Electrochemistry Voltage Electrochemistry is an interfacial science between. one volt (v) is the potential difference necessary to generate a charge of 1 coulomb (c) from 1 joule (j) of energy. For a voltaic cell, this potential difference is called the cell potential (or emf for electromotive force, although it is not really a force), which is denoted ecell. the galvanic. Electrochemistry Voltage.
From saylordotorg.github.io
Describing Electrochemical Cells Electrochemistry Voltage the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. the cell potential is the way in which we can measure how much voltage exists between the two half cells of a battery. one volt (v) is the potential difference necessary to generate a charge of 1. Electrochemistry Voltage.
From www.researchgate.net
(a) Electrochemical voltagecapacity curve obtained during Mg cycling Electrochemistry Voltage the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. a voltmeter measures the difference in the electrochemical potential of electrons (μ̅ e), because it is this difference that drives the current. For a voltaic cell, this potential difference is called the cell potential (or emf for electromotive. Electrochemistry Voltage.
From www.youtube.com
BCLN Electrochemical CellsFinding Initial Voltage Chemistry YouTube Electrochemistry Voltage a voltmeter measures the difference in the electrochemical potential of electrons (μ̅ e), because it is this difference that drives the current. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. one volt (v) is the potential difference necessary to generate a charge of 1 coulomb. Electrochemistry Voltage.
From slideplayer.com
Electrochemistry Generating Voltage (Potential) ppt download Electrochemistry Voltage For a voltaic cell, this potential difference is called the cell potential (or emf for electromotive force, although it is not really a force), which is denoted ecell. a voltmeter measures the difference in the electrochemical potential of electrons (μ̅ e), because it is this difference that drives the current. one volt (v) is the potential difference necessary. Electrochemistry Voltage.
From www.dynamicscience.com.au
redox reactions electrolysis The difference between electrochemical Electrochemistry Voltage Electrochemistry is an interfacial science between. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. For a voltaic cell, this potential difference is called the cell potential (or emf for electromotive force, although it is not really a force), which is denoted ecell. one volt (v) is. Electrochemistry Voltage.
From www.youtube.com
Calculating the Cell Potential of Electrochemical Cells. (Adv Chem Ch Electrochemistry Voltage the cell potential is the way in which we can measure how much voltage exists between the two half cells of a battery. a voltmeter measures the difference in the electrochemical potential of electrons (μ̅ e), because it is this difference that drives the current. one volt (v) is the potential difference necessary to generate a charge. Electrochemistry Voltage.
From www.researchgate.net
a Electrochemical voltage stability window for different GPEs Electrochemistry Voltage For a voltaic cell, this potential difference is called the cell potential (or emf for electromotive force, although it is not really a force), which is denoted ecell. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. one volt (v) is the potential difference necessary to generate. Electrochemistry Voltage.
From unacademy.com
Electrochemical Series, Features and Importance Unacademy Electrochemistry Voltage Electrochemistry is an interfacial science between. one volt (v) is the potential difference necessary to generate a charge of 1 coulomb (c) from 1 joule (j) of energy. the cell potential is the way in which we can measure how much voltage exists between the two half cells of a battery. the standard cell potential for a. Electrochemistry Voltage.
From www.researchgate.net
Electrochemistry tests for Li⁺ transport and high‐voltage/cycle Electrochemistry Voltage For a voltaic cell, this potential difference is called the cell potential (or emf for electromotive force, although it is not really a force), which is denoted ecell. the standard cell potential for a redox reaction (e° cell) is a measure of the tendency of reactants in their standard. a voltmeter measures the difference in the electrochemical potential. Electrochemistry Voltage.
From www.researchgate.net
Schematic showing the description of voltagedependent electrochemical Electrochemistry Voltage For a voltaic cell, this potential difference is called the cell potential (or emf for electromotive force, although it is not really a force), which is denoted ecell. one volt (v) is the potential difference necessary to generate a charge of 1 coulomb (c) from 1 joule (j) of energy. Electrochemistry is an interfacial science between. the standard. Electrochemistry Voltage.
From leverageedu.com
Electrochemical Series Notes Chemistry Class 11 & 12 Leverage Edu Electrochemistry Voltage For a voltaic cell, this potential difference is called the cell potential (or emf for electromotive force, although it is not really a force), which is denoted ecell. the standard cell potential for a redox reaction (e° cell) is a measure of the tendency of reactants in their standard. Electrochemistry is an interfacial science between. the cell potential. Electrochemistry Voltage.
From www.youtube.com
Write note on Hydrogen Over Voltage? Applied Electrochemistry Electrochemistry Voltage For a voltaic cell, this potential difference is called the cell potential (or emf for electromotive force, although it is not really a force), which is denoted ecell. the cell potential is the way in which we can measure how much voltage exists between the two half cells of a battery. the galvanic cell, or called voltaic cell,. Electrochemistry Voltage.
From courses.lumenlearning.com
Electrolysis Chemistry Electrochemistry Voltage the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. a voltmeter measures the difference in the electrochemical potential of electrons (μ̅ e), because it is this difference that drives the current. the cell potential is the way in which we can measure how much voltage exists. Electrochemistry Voltage.
From www.chemistrystudent.com
Electrochemical Cells (ALevel) ChemistryStudent Electrochemistry Voltage the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. For a voltaic cell, this potential difference is called the cell potential (or emf for electromotive force, although it is not really a force), which is denoted ecell. the standard cell potential for a redox reaction (e° cell). Electrochemistry Voltage.
From www.youtube.com
What Is The Electrochemical Series Reactions Chemistry FuseSchool Electrochemistry Voltage Electrochemistry is an interfacial science between. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. one volt (v) is the potential difference necessary to generate a charge of 1 coulomb (c) from 1 joule (j) of energy. the standard cell potential for a redox reaction (e°. Electrochemistry Voltage.
From cepwqyvd.blob.core.windows.net
What Is Electrochemical Properties at Elizabeth Watkins blog Electrochemistry Voltage Electrochemistry is an interfacial science between. the standard cell potential for a redox reaction (e° cell) is a measure of the tendency of reactants in their standard. For a voltaic cell, this potential difference is called the cell potential (or emf for electromotive force, although it is not really a force), which is denoted ecell. one volt (v). Electrochemistry Voltage.
From www.researchgate.net
a) Electrochemical voltage spectroscopy (EVS) data for a typical Electrochemistry Voltage a voltmeter measures the difference in the electrochemical potential of electrons (μ̅ e), because it is this difference that drives the current. one volt (v) is the potential difference necessary to generate a charge of 1 coulomb (c) from 1 joule (j) of energy. For a voltaic cell, this potential difference is called the cell potential (or emf. Electrochemistry Voltage.
From electricalacademia.com
Voltaic Cell Working and Construction of Voltaic Cell Electrical Electrochemistry Voltage For a voltaic cell, this potential difference is called the cell potential (or emf for electromotive force, although it is not really a force), which is denoted ecell. the standard cell potential for a redox reaction (e° cell) is a measure of the tendency of reactants in their standard. one volt (v) is the potential difference necessary to. Electrochemistry Voltage.
From studylib.net
INTRODUCTION TO ELECTROCHEMISTRY CURRENT, VOLTAGE Electrochemistry Voltage For a voltaic cell, this potential difference is called the cell potential (or emf for electromotive force, although it is not really a force), which is denoted ecell. one volt (v) is the potential difference necessary to generate a charge of 1 coulomb (c) from 1 joule (j) of energy. the galvanic cell, or called voltaic cell, is. Electrochemistry Voltage.
From www.yaclass.in
Types of Electrochemical Cell and Electrolytic Cell — lesson. Science Electrochemistry Voltage one volt (v) is the potential difference necessary to generate a charge of 1 coulomb (c) from 1 joule (j) of energy. a voltmeter measures the difference in the electrochemical potential of electrons (μ̅ e), because it is this difference that drives the current. the standard cell potential for a redox reaction (e° cell) is a measure. Electrochemistry Voltage.
From chem.libretexts.org
Voltaic Cells Chemistry LibreTexts Electrochemistry Voltage the cell potential is the way in which we can measure how much voltage exists between the two half cells of a battery. Electrochemistry is an interfacial science between. a voltmeter measures the difference in the electrochemical potential of electrons (μ̅ e), because it is this difference that drives the current. the standard cell potential for a. Electrochemistry Voltage.
From www.youtube.com
How To Draw Galvanic Cells and Voltaic Cells Electrochemistry YouTube Electrochemistry Voltage one volt (v) is the potential difference necessary to generate a charge of 1 coulomb (c) from 1 joule (j) of energy. a voltmeter measures the difference in the electrochemical potential of electrons (μ̅ e), because it is this difference that drives the current. Electrochemistry is an interfacial science between. the galvanic cell, or called voltaic cell,. Electrochemistry Voltage.
From www.slideserve.com
PPT Electric power conversion in electrochemistry PowerPoint Electrochemistry Voltage the cell potential is the way in which we can measure how much voltage exists between the two half cells of a battery. a voltmeter measures the difference in the electrochemical potential of electrons (μ̅ e), because it is this difference that drives the current. the standard cell potential for a redox reaction (e° cell) is a. Electrochemistry Voltage.
From www.slideserve.com
PPT Electrochemistry Generating Voltage (Potential) PowerPoint Electrochemistry Voltage the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. the cell potential is the way in which we can measure how much voltage exists between the two half cells of a battery. For a voltaic cell, this potential difference is called the cell potential (or emf for. Electrochemistry Voltage.
From 2012books.lardbucket.org
Electrochemistry Electrochemistry Voltage For a voltaic cell, this potential difference is called the cell potential (or emf for electromotive force, although it is not really a force), which is denoted ecell. Electrochemistry is an interfacial science between. a voltmeter measures the difference in the electrochemical potential of electrons (μ̅ e), because it is this difference that drives the current. one volt. Electrochemistry Voltage.
From www.youtube.com
Cell Potential Problems Electrochemistry YouTube Electrochemistry Voltage the standard cell potential for a redox reaction (e° cell) is a measure of the tendency of reactants in their standard. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. the cell potential is the way in which we can measure how much voltage exists between. Electrochemistry Voltage.
From www.studocu.com
Electrochemistry Lab Chem 1B Electrochemistry Experiment 1 Standard Electrochemistry Voltage one volt (v) is the potential difference necessary to generate a charge of 1 coulomb (c) from 1 joule (j) of energy. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. For a voltaic cell, this potential difference is called the cell potential (or emf for electromotive. Electrochemistry Voltage.
From www.sigmaaldrich.com
Electrochemistry on the Bench and in the Field Electrochemistry Voltage the cell potential is the way in which we can measure how much voltage exists between the two half cells of a battery. Electrochemistry is an interfacial science between. a voltmeter measures the difference in the electrochemical potential of electrons (μ̅ e), because it is this difference that drives the current. the galvanic cell, or called voltaic. Electrochemistry Voltage.
From www.youtube.com
KAC32.7 Electrochemistry Effect of Concentration on Cell Potentials Electrochemistry Voltage Electrochemistry is an interfacial science between. For a voltaic cell, this potential difference is called the cell potential (or emf for electromotive force, although it is not really a force), which is denoted ecell. one volt (v) is the potential difference necessary to generate a charge of 1 coulomb (c) from 1 joule (j) of energy. the standard. Electrochemistry Voltage.
From chem.libretexts.org
Chapter 19.1 Describing Electrochemical Cells Chemistry LibreTexts Electrochemistry Voltage For a voltaic cell, this potential difference is called the cell potential (or emf for electromotive force, although it is not really a force), which is denoted ecell. Electrochemistry is an interfacial science between. the standard cell potential for a redox reaction (e° cell) is a measure of the tendency of reactants in their standard. the cell potential. Electrochemistry Voltage.