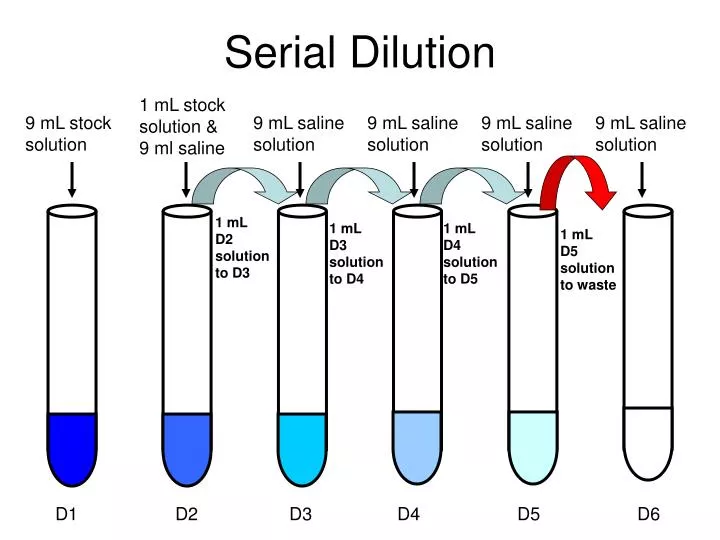
Titration Calculations With Dilution . A 25 cm3 sample of. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in [link]). • proper use of a pipette and a. Titration calculations are used to find the concentration of unknown solutions; They can also be used to calculate the ph after a given point during a titration By using a solution with a. Use the information to determine the concentration of the hydrochloric acid. Calculate the ph at these volumes of added base solution: The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. The specific skills relevant to this procedure are: For example if we are asked to find a purity of the substance, we must convert concentration found to amount of substance in.
from wiener.me
• proper use of a pipette and a. The specific skills relevant to this procedure are: They can also be used to calculate the ph after a given point during a titration The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. A 25 cm3 sample of. Calculate the ph at these volumes of added base solution: Titration calculations are used to find the concentration of unknown solutions; Use the information to determine the concentration of the hydrochloric acid. For example if we are asked to find a purity of the substance, we must convert concentration found to amount of substance in. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in [link]).
Dilution Techniques And Calculations, 44 OFF wiener.me
Titration Calculations With Dilution A 25 cm3 sample of. The specific skills relevant to this procedure are: • proper use of a pipette and a. A 25 cm3 sample of. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in [link]). They can also be used to calculate the ph after a given point during a titration Calculate the ph at these volumes of added base solution: Titration calculations are used to find the concentration of unknown solutions; The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. For example if we are asked to find a purity of the substance, we must convert concentration found to amount of substance in. By using a solution with a. Use the information to determine the concentration of the hydrochloric acid.
From www.studypool.com
SOLUTION Titration calculations and answers Studypool Titration Calculations With Dilution Use the information to determine the concentration of the hydrochloric acid. A 25 cm3 sample of. They can also be used to calculate the ph after a given point during a titration By using a solution with a. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh. Titration Calculations With Dilution.
From printablehaferbrotwp.z21.web.core.windows.net
How To Do Titrations In Chemistry Titration Calculations With Dilution The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. A 25 cm3 sample of. By using a solution with a. Use the information to determine the concentration of the hydrochloric acid. The specific skills relevant to this procedure are: For example if we are asked to find a purity. Titration Calculations With Dilution.
From www.youtube.com
Titration of Mohr Salt Solution with Standard Potassium Dichromate Titration Calculations With Dilution The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. Use the information to determine the concentration of the hydrochloric acid. For example if we are asked to find a purity of the substance, we must convert concentration found to amount of substance in. Titration calculations are used to find. Titration Calculations With Dilution.
From www.youtube.com
Serial Dilution Method Protocol Step Wise Explanation YouTube Titration Calculations With Dilution Calculate the ph at these volumes of added base solution: By using a solution with a. Titration calculations are used to find the concentration of unknown solutions; They can also be used to calculate the ph after a given point during a titration For example if we are asked to find a purity of the substance, we must convert concentration. Titration Calculations With Dilution.
From www.science-revision.co.uk
Titrations Titration Calculations With Dilution A 25 cm3 sample of. Use the information to determine the concentration of the hydrochloric acid. For example if we are asked to find a purity of the substance, we must convert concentration found to amount of substance in. Calculate the ph at these volumes of added base solution: A titration is carried out for 25.00 ml of 0.100 m. Titration Calculations With Dilution.
From www.youtube.com
Chemistry Concepts Dilution and Titration Calculations.mp4 YouTube Titration Calculations With Dilution The specific skills relevant to this procedure are: The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. A 25 cm3 sample of. Use the information to determine the concentration of the hydrochloric acid. Calculate the ph at these volumes of added base solution: By using a solution with a.. Titration Calculations With Dilution.
From www.slideserve.com
PPT Chapter 8 Solutions PowerPoint Presentation, free download ID Titration Calculations With Dilution Titration calculations are used to find the concentration of unknown solutions; • proper use of a pipette and a. They can also be used to calculate the ph after a given point during a titration A 25 cm3 sample of. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide.. Titration Calculations With Dilution.
From www.youtube.com
Titration calculation involving dilution and sampling (A Level Titration Calculations With Dilution • proper use of a pipette and a. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. For example if we are asked to find a purity of the substance, we must convert concentration found to amount of substance in. By using a solution with a. Titration calculations are. Titration Calculations With Dilution.
From noel-kmclean.blogspot.com
Calculate Molarity of Acid in Titration Titration Calculations With Dilution The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. Calculate the ph at these volumes of added base solution: For example if we are asked to find a purity of the substance, we must convert concentration found to amount of substance in. A 25 cm3 sample of. By using. Titration Calculations With Dilution.
From www.youtube.com
Quickvideo Using the dilution equation M1V1=M2V2 YouTube Titration Calculations With Dilution The specific skills relevant to this procedure are: Use the information to determine the concentration of the hydrochloric acid. Titration calculations are used to find the concentration of unknown solutions; The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. A 25 cm3 sample of. A titration is carried out. Titration Calculations With Dilution.
From www.youtube.com
DilutionTitration Calculation ChemCram 101 Tutorial YouTube Titration Calculations With Dilution The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. They can also be used to calculate the ph after a given point during a titration Use the information to determine the concentration of the hydrochloric acid. A 25 cm3 sample of. By using a solution with a. • proper. Titration Calculations With Dilution.
From www.youtube.com
VA06 Dilution and Aliquot Calculations Year 12 WACE Chemistry YouTube Titration Calculations With Dilution Use the information to determine the concentration of the hydrochloric acid. By using a solution with a. Titration calculations are used to find the concentration of unknown solutions; Calculate the ph at these volumes of added base solution: The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. They can. Titration Calculations With Dilution.
From www.youtube.com
How to Do Titration Calculations // HSC Chemistry YouTube Titration Calculations With Dilution The specific skills relevant to this procedure are: A 25 cm3 sample of. For example if we are asked to find a purity of the substance, we must convert concentration found to amount of substance in. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. Calculate the ph at. Titration Calculations With Dilution.
From www.sliderbase.com
Dilution Titration Calculations With Dilution Titration calculations are used to find the concentration of unknown solutions; The specific skills relevant to this procedure are: A 25 cm3 sample of. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. Calculate the ph at these volumes of added base solution: A titration is carried out for. Titration Calculations With Dilution.
From loeppodiz.blob.core.windows.net
Titration Calculation Step By Step Pdf at Linda Tibbetts blog Titration Calculations With Dilution The specific skills relevant to this procedure are: Use the information to determine the concentration of the hydrochloric acid. By using a solution with a. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in [link]). Titration calculations are used to find. Titration Calculations With Dilution.
From www.wikihow.com
5 Easy Ways to Calculate the Concentration of a Solution Titration Calculations With Dilution By using a solution with a. Calculate the ph at these volumes of added base solution: The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. The specific skills relevant to this procedure are: Titration calculations are used to find the concentration of unknown solutions; A 25 cm3 sample of.. Titration Calculations With Dilution.
From www.youtube.com
Acid Base Titration Problems, Basic Introduction, Calculations Titration Calculations With Dilution For example if we are asked to find a purity of the substance, we must convert concentration found to amount of substance in. Calculate the ph at these volumes of added base solution: A 25 cm3 sample of. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh. Titration Calculations With Dilution.
From wiener.me
Dilution Techniques And Calculations, 44 OFF wiener.me Titration Calculations With Dilution They can also be used to calculate the ph after a given point during a titration Calculate the ph at these volumes of added base solution: The specific skills relevant to this procedure are: The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. • proper use of a pipette. Titration Calculations With Dilution.
From mungfali.com
Acid Base Titration Procedure Titration Calculations With Dilution Titration calculations are used to find the concentration of unknown solutions; Calculate the ph at these volumes of added base solution: For example if we are asked to find a purity of the substance, we must convert concentration found to amount of substance in. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100. Titration Calculations With Dilution.
From www.youtube.com
Higher Chemistry Calculations Titrations YouTube Titration Calculations With Dilution A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in [link]). They can also be used to calculate the ph after a given point during a titration Titration calculations are used to find the concentration of unknown solutions; By using a solution. Titration Calculations With Dilution.
From mungfali.com
Acid Base Titration Calculation Titration Calculations With Dilution The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in [link]). For example if we are asked to find a purity of. Titration Calculations With Dilution.
From knowledge.carolina.com
Titrations Techniques and Calculations Carolina Knowledge Center Titration Calculations With Dilution A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in [link]). A 25 cm3 sample of. For example if we are asked to find a purity of the substance, we must convert concentration found to amount of substance in. • proper use. Titration Calculations With Dilution.
From ar.inspiredpencil.com
Titration Problem Examples Titration Calculations With Dilution Use the information to determine the concentration of the hydrochloric acid. A 25 cm3 sample of. For example if we are asked to find a purity of the substance, we must convert concentration found to amount of substance in. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. By. Titration Calculations With Dilution.
From dxopazssz.blob.core.windows.net
Dilution Calculator From Mass at Blanca Norton blog Titration Calculations With Dilution Calculate the ph at these volumes of added base solution: A 25 cm3 sample of. Titration calculations are used to find the concentration of unknown solutions; The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. The specific skills relevant to this procedure are: By using a solution with a.. Titration Calculations With Dilution.
From mavink.com
Acid Base Titration Equation Titration Calculations With Dilution • proper use of a pipette and a. They can also be used to calculate the ph after a given point during a titration Titration calculations are used to find the concentration of unknown solutions; Use the information to determine the concentration of the hydrochloric acid. By using a solution with a. Calculate the ph at these volumes of added. Titration Calculations With Dilution.
From www.slideserve.com
PPT Dilution Calculations PowerPoint Presentation, free download ID Titration Calculations With Dilution Titration calculations are used to find the concentration of unknown solutions; • proper use of a pipette and a. A 25 cm3 sample of. Calculate the ph at these volumes of added base solution: The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. Use the information to determine the. Titration Calculations With Dilution.
From www.studocu.com
Experiment 3 lectures DoubleIndicator Titration Method ACIDBASE Titration Calculations With Dilution The specific skills relevant to this procedure are: They can also be used to calculate the ph after a given point during a titration The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. Calculate the ph at these volumes of added base solution: By using a solution with a.. Titration Calculations With Dilution.
From www.youtube.com
Dilution Calculation Practice YouTube Titration Calculations With Dilution Calculate the ph at these volumes of added base solution: For example if we are asked to find a purity of the substance, we must convert concentration found to amount of substance in. Titration calculations are used to find the concentration of unknown solutions; A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100. Titration Calculations With Dilution.
From celtjcvb.blob.core.windows.net
Titration In Simple Terms at John Haslett blog Titration Calculations With Dilution For example if we are asked to find a purity of the substance, we must convert concentration found to amount of substance in. Titration calculations are used to find the concentration of unknown solutions; The specific skills relevant to this procedure are: A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of. Titration Calculations With Dilution.
From www.youtube.com
How to calculate molarity from titration data? Stock Solution vs Titration Calculations With Dilution By using a solution with a. • proper use of a pipette and a. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in [link]). Titration calculations are used to find the concentration of unknown solutions; A 25 cm3 sample of. Calculate. Titration Calculations With Dilution.
From borenew.weebly.com
Serial Dilution Calculation Examples borenew Titration Calculations With Dilution The specific skills relevant to this procedure are: They can also be used to calculate the ph after a given point during a titration A 25 cm3 sample of. Calculate the ph at these volumes of added base solution: Use the information to determine the concentration of the hydrochloric acid. • proper use of a pipette and a. For example. Titration Calculations With Dilution.
From www.slideserve.com
PPT TITRATION PowerPoint Presentation, free download ID1459481 Titration Calculations With Dilution The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. Calculate the ph at these volumes of added base solution: Use the information to determine the concentration of the hydrochloric acid. For example if we are asked to find a purity of the substance, we must convert concentration found to. Titration Calculations With Dilution.
From www.researchgate.net
The sample, dilution factor, and BOD values and the calculated degrees Titration Calculations With Dilution Titration calculations are used to find the concentration of unknown solutions; A 25 cm3 sample of. They can also be used to calculate the ph after a given point during a titration A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in. Titration Calculations With Dilution.
From mungfali.com
Acid Base Titration Formula Titration Calculations With Dilution The specific skills relevant to this procedure are: Titration calculations are used to find the concentration of unknown solutions; They can also be used to calculate the ph after a given point during a titration For example if we are asked to find a purity of the substance, we must convert concentration found to amount of substance in. • proper. Titration Calculations With Dilution.
From www.sliderbase.com
Recording in titration Titration Calculations With Dilution A 25 cm3 sample of. Use the information to determine the concentration of the hydrochloric acid. The specific skills relevant to this procedure are: • proper use of a pipette and a. By using a solution with a. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh. Titration Calculations With Dilution.