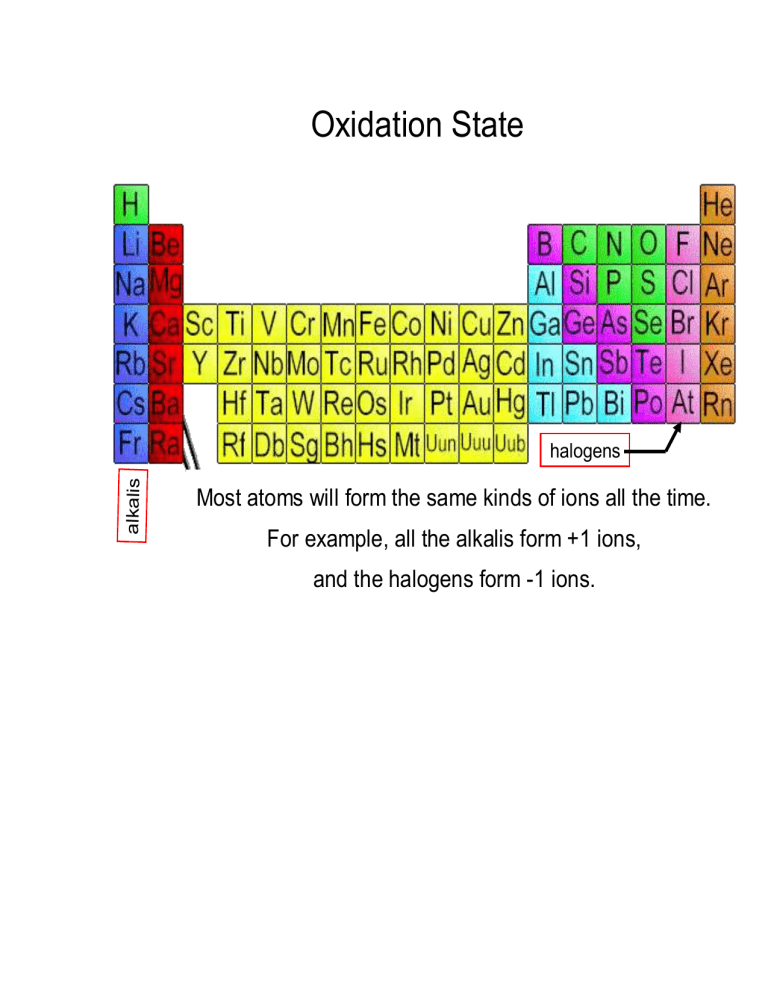
What Is The Oxidation Number Of N In Zn(No3)2 . What is the oxidation number of each element in each of the following substances? C120 the oxidation number of cl is the oxidation number of o is b. Enter the formula of a chemical compound to find the oxidation number of each element. How to find the oxidation numbers for zn (no3)2 (zinc nitrate) to find the correct oxidation state of in. A net ionic charge can be specified at the end of the. The oxidation number of each atom can be calculated by subtracting the sum of lone pairs and electrons it gains from bonds from the number of valence electrons. In zn(no3) 2 , the oxidation number of only zn is +2. Balance the following redox reaction by oxidation number method: Zn goes from 0 to +2 and n. Bonds between atoms of the same. So the oxidation numer of zn (no3)2 = 0. This is a redox reaction and you should start by writing the oxidation and reduction halves and balancing them.
from studylib.net
Balance the following redox reaction by oxidation number method: Enter the formula of a chemical compound to find the oxidation number of each element. In zn(no3) 2 , the oxidation number of only zn is +2. How to find the oxidation numbers for zn (no3)2 (zinc nitrate) to find the correct oxidation state of in. Zn goes from 0 to +2 and n. A net ionic charge can be specified at the end of the. What is the oxidation number of each element in each of the following substances? This is a redox reaction and you should start by writing the oxidation and reduction halves and balancing them. Bonds between atoms of the same. C120 the oxidation number of cl is the oxidation number of o is b.
Oxidation Numbers
What Is The Oxidation Number Of N In Zn(No3)2 In zn(no3) 2 , the oxidation number of only zn is +2. C120 the oxidation number of cl is the oxidation number of o is b. A net ionic charge can be specified at the end of the. The oxidation number of each atom can be calculated by subtracting the sum of lone pairs and electrons it gains from bonds from the number of valence electrons. So the oxidation numer of zn (no3)2 = 0. Enter the formula of a chemical compound to find the oxidation number of each element. How to find the oxidation numbers for zn (no3)2 (zinc nitrate) to find the correct oxidation state of in. Zn goes from 0 to +2 and n. This is a redox reaction and you should start by writing the oxidation and reduction halves and balancing them. Balance the following redox reaction by oxidation number method: What is the oxidation number of each element in each of the following substances? In zn(no3) 2 , the oxidation number of only zn is +2. Bonds between atoms of the same.
From www.toppr.com
Complete and balance the given reaction. Zn + NO3^ Zn^2 + + NH4^+ What Is The Oxidation Number Of N In Zn(No3)2 How to find the oxidation numbers for zn (no3)2 (zinc nitrate) to find the correct oxidation state of in. A net ionic charge can be specified at the end of the. Balance the following redox reaction by oxidation number method: What is the oxidation number of each element in each of the following substances? The oxidation number of each atom. What Is The Oxidation Number Of N In Zn(No3)2.
From quizlet.com
periodic table oxidation Numbers Diagram Quizlet What Is The Oxidation Number Of N In Zn(No3)2 How to find the oxidation numbers for zn (no3)2 (zinc nitrate) to find the correct oxidation state of in. The oxidation number of each atom can be calculated by subtracting the sum of lone pairs and electrons it gains from bonds from the number of valence electrons. Balance the following redox reaction by oxidation number method: This is a redox. What Is The Oxidation Number Of N In Zn(No3)2.
From www.slideserve.com
PPT Oxidation Numbers PowerPoint Presentation, free download ID5075015 What Is The Oxidation Number Of N In Zn(No3)2 The oxidation number of each atom can be calculated by subtracting the sum of lone pairs and electrons it gains from bonds from the number of valence electrons. This is a redox reaction and you should start by writing the oxidation and reduction halves and balancing them. Zn goes from 0 to +2 and n. What is the oxidation number. What Is The Oxidation Number Of N In Zn(No3)2.
From www.youtube.com
How to find the Oxidation Number for N in Mg(NO3)2 (Magnesium nitrate What Is The Oxidation Number Of N In Zn(No3)2 What is the oxidation number of each element in each of the following substances? This is a redox reaction and you should start by writing the oxidation and reduction halves and balancing them. In zn(no3) 2 , the oxidation number of only zn is +2. So the oxidation numer of zn (no3)2 = 0. Enter the formula of a chemical. What Is The Oxidation Number Of N In Zn(No3)2.
From www.youtube.com
How to Write the Net Ionic Equation for Zn + Sn(NO3)2= Zn(NO3)2 + Sn What Is The Oxidation Number Of N In Zn(No3)2 Zn goes from 0 to +2 and n. Bonds between atoms of the same. Enter the formula of a chemical compound to find the oxidation number of each element. This is a redox reaction and you should start by writing the oxidation and reduction halves and balancing them. So the oxidation numer of zn (no3)2 = 0. A net ionic. What Is The Oxidation Number Of N In Zn(No3)2.
From www.slideserve.com
PPT Oxidation Numbers PowerPoint Presentation, free download ID5075015 What Is The Oxidation Number Of N In Zn(No3)2 Balance the following redox reaction by oxidation number method: A net ionic charge can be specified at the end of the. Enter the formula of a chemical compound to find the oxidation number of each element. Bonds between atoms of the same. Zn goes from 0 to +2 and n. C120 the oxidation number of cl is the oxidation number. What Is The Oxidation Number Of N In Zn(No3)2.
From www.chegg.com
Solved 1. Determine the oxidation number of the elements in What Is The Oxidation Number Of N In Zn(No3)2 Balance the following redox reaction by oxidation number method: How to find the oxidation numbers for zn (no3)2 (zinc nitrate) to find the correct oxidation state of in. Bonds between atoms of the same. The oxidation number of each atom can be calculated by subtracting the sum of lone pairs and electrons it gains from bonds from the number of. What Is The Oxidation Number Of N In Zn(No3)2.
From www.slideserve.com
PPT Oxidation Numbers PowerPoint Presentation, free download ID5075015 What Is The Oxidation Number Of N In Zn(No3)2 Zn goes from 0 to +2 and n. The oxidation number of each atom can be calculated by subtracting the sum of lone pairs and electrons it gains from bonds from the number of valence electrons. Bonds between atoms of the same. Enter the formula of a chemical compound to find the oxidation number of each element. A net ionic. What Is The Oxidation Number Of N In Zn(No3)2.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID2395087 What Is The Oxidation Number Of N In Zn(No3)2 A net ionic charge can be specified at the end of the. Enter the formula of a chemical compound to find the oxidation number of each element. So the oxidation numer of zn (no3)2 = 0. C120 the oxidation number of cl is the oxidation number of o is b. In zn(no3) 2 , the oxidation number of only zn. What Is The Oxidation Number Of N In Zn(No3)2.
From www.onlinechemistrytutor.net
Oxidation state examples Online Chemistry Tutor What Is The Oxidation Number Of N In Zn(No3)2 This is a redox reaction and you should start by writing the oxidation and reduction halves and balancing them. In zn(no3) 2 , the oxidation number of only zn is +2. The oxidation number of each atom can be calculated by subtracting the sum of lone pairs and electrons it gains from bonds from the number of valence electrons. Zn. What Is The Oxidation Number Of N In Zn(No3)2.
From www.toppr.com
For the redox reaction Zn + NO^ 3→ Zn^2 + + NH^ + 4 in a basic What Is The Oxidation Number Of N In Zn(No3)2 In zn(no3) 2 , the oxidation number of only zn is +2. Zn goes from 0 to +2 and n. Enter the formula of a chemical compound to find the oxidation number of each element. What is the oxidation number of each element in each of the following substances? Bonds between atoms of the same. C120 the oxidation number of. What Is The Oxidation Number Of N In Zn(No3)2.
From www.youtube.com
How to Draw the Lewis Dot Structure for Zn(NO3)2 Zinc nitrate YouTube What Is The Oxidation Number Of N In Zn(No3)2 Enter the formula of a chemical compound to find the oxidation number of each element. This is a redox reaction and you should start by writing the oxidation and reduction halves and balancing them. What is the oxidation number of each element in each of the following substances? C120 the oxidation number of cl is the oxidation number of o. What Is The Oxidation Number Of N In Zn(No3)2.
From www.youtube.com
How to find the Oxidation Number for Zn (Zinc) YouTube What Is The Oxidation Number Of N In Zn(No3)2 How to find the oxidation numbers for zn (no3)2 (zinc nitrate) to find the correct oxidation state of in. This is a redox reaction and you should start by writing the oxidation and reduction halves and balancing them. The oxidation number of each atom can be calculated by subtracting the sum of lone pairs and electrons it gains from bonds. What Is The Oxidation Number Of N In Zn(No3)2.
From www.youtube.com
How to Balance Zn(NO3)2 = ZnO + NO2 + O2 (Zinc Nitrate What Is The Oxidation Number Of N In Zn(No3)2 Bonds between atoms of the same. In zn(no3) 2 , the oxidation number of only zn is +2. The oxidation number of each atom can be calculated by subtracting the sum of lone pairs and electrons it gains from bonds from the number of valence electrons. Balance the following redox reaction by oxidation number method: C120 the oxidation number of. What Is The Oxidation Number Of N In Zn(No3)2.
From www.slideserve.com
PPT Oxidation Numbers PowerPoint Presentation, free download ID5075015 What Is The Oxidation Number Of N In Zn(No3)2 Zn goes from 0 to +2 and n. What is the oxidation number of each element in each of the following substances? Enter the formula of a chemical compound to find the oxidation number of each element. Bonds between atoms of the same. A net ionic charge can be specified at the end of the. Balance the following redox reaction. What Is The Oxidation Number Of N In Zn(No3)2.
From www.youtube.com
How to find the Oxidation Numbers for Zn(NO3)2 (Zinc nitrate) YouTube What Is The Oxidation Number Of N In Zn(No3)2 A net ionic charge can be specified at the end of the. Balance the following redox reaction by oxidation number method: So the oxidation numer of zn (no3)2 = 0. C120 the oxidation number of cl is the oxidation number of o is b. How to find the oxidation numbers for zn (no3)2 (zinc nitrate) to find the correct oxidation. What Is The Oxidation Number Of N In Zn(No3)2.
From www.youtube.com
How to calculate the oxidation number of Nitrogen in N2O(Nitrous oxide What Is The Oxidation Number Of N In Zn(No3)2 The oxidation number of each atom can be calculated by subtracting the sum of lone pairs and electrons it gains from bonds from the number of valence electrons. This is a redox reaction and you should start by writing the oxidation and reduction halves and balancing them. What is the oxidation number of each element in each of the following. What Is The Oxidation Number Of N In Zn(No3)2.
From www.youtube.com
How to Write the Net Ionic Equation for Mg + Zn(NO3)2 = Mg(NO3)2 + Zn What Is The Oxidation Number Of N In Zn(No3)2 Bonds between atoms of the same. How to find the oxidation numbers for zn (no3)2 (zinc nitrate) to find the correct oxidation state of in. A net ionic charge can be specified at the end of the. Enter the formula of a chemical compound to find the oxidation number of each element. Balance the following redox reaction by oxidation number. What Is The Oxidation Number Of N In Zn(No3)2.
From www.slideserve.com
PPT 13.3b Balancing equations using oxidation numbers PowerPoint What Is The Oxidation Number Of N In Zn(No3)2 A net ionic charge can be specified at the end of the. C120 the oxidation number of cl is the oxidation number of o is b. Bonds between atoms of the same. The oxidation number of each atom can be calculated by subtracting the sum of lone pairs and electrons it gains from bonds from the number of valence electrons.. What Is The Oxidation Number Of N In Zn(No3)2.
From www.numerade.com
SOLVED A concentration cell consists of two Zn/Zn2+ electrodes The What Is The Oxidation Number Of N In Zn(No3)2 How to find the oxidation numbers for zn (no3)2 (zinc nitrate) to find the correct oxidation state of in. Enter the formula of a chemical compound to find the oxidation number of each element. C120 the oxidation number of cl is the oxidation number of o is b. A net ionic charge can be specified at the end of the.. What Is The Oxidation Number Of N In Zn(No3)2.
From www.youtube.com
How to find the Oxidation Number for N in NO3 (Nitrate ion) YouTube What Is The Oxidation Number Of N In Zn(No3)2 A net ionic charge can be specified at the end of the. What is the oxidation number of each element in each of the following substances? C120 the oxidation number of cl is the oxidation number of o is b. This is a redox reaction and you should start by writing the oxidation and reduction halves and balancing them. Zn. What Is The Oxidation Number Of N In Zn(No3)2.
From www.slideserve.com
PPT 9. OxidationReduction PowerPoint Presentation, free download What Is The Oxidation Number Of N In Zn(No3)2 Zn goes from 0 to +2 and n. C120 the oxidation number of cl is the oxidation number of o is b. In zn(no3) 2 , the oxidation number of only zn is +2. Enter the formula of a chemical compound to find the oxidation number of each element. This is a redox reaction and you should start by writing. What Is The Oxidation Number Of N In Zn(No3)2.
From www.slideserve.com
PPT Assigning Oxidation Numbers PowerPoint Presentation, free What Is The Oxidation Number Of N In Zn(No3)2 Balance the following redox reaction by oxidation number method: C120 the oxidation number of cl is the oxidation number of o is b. In zn(no3) 2 , the oxidation number of only zn is +2. Bonds between atoms of the same. This is a redox reaction and you should start by writing the oxidation and reduction halves and balancing them.. What Is The Oxidation Number Of N In Zn(No3)2.
From www.slideserve.com
PPT 7.3 Balancing Redox Reactions Using Oxidation Numbers What Is The Oxidation Number Of N In Zn(No3)2 So the oxidation numer of zn (no3)2 = 0. C120 the oxidation number of cl is the oxidation number of o is b. This is a redox reaction and you should start by writing the oxidation and reduction halves and balancing them. In zn(no3) 2 , the oxidation number of only zn is +2. Zn goes from 0 to +2. What Is The Oxidation Number Of N In Zn(No3)2.
From www.chegg.com
Solved The reaction Zn(s) + Cu(NO3)2(aq) → Zn(NO3)2(aq) + What Is The Oxidation Number Of N In Zn(No3)2 Enter the formula of a chemical compound to find the oxidation number of each element. This is a redox reaction and you should start by writing the oxidation and reduction halves and balancing them. In zn(no3) 2 , the oxidation number of only zn is +2. The oxidation number of each atom can be calculated by subtracting the sum of. What Is The Oxidation Number Of N In Zn(No3)2.
From www.toppr.com
For the redox reaction Zn + NO^ 3→ Zn^2 + + NH^ + 4 in a basic What Is The Oxidation Number Of N In Zn(No3)2 What is the oxidation number of each element in each of the following substances? Enter the formula of a chemical compound to find the oxidation number of each element. In zn(no3) 2 , the oxidation number of only zn is +2. The oxidation number of each atom can be calculated by subtracting the sum of lone pairs and electrons it. What Is The Oxidation Number Of N In Zn(No3)2.
From www.slideserve.com
PPT Oxidation Numbers PowerPoint Presentation, free download ID5075015 What Is The Oxidation Number Of N In Zn(No3)2 Bonds between atoms of the same. What is the oxidation number of each element in each of the following substances? The oxidation number of each atom can be calculated by subtracting the sum of lone pairs and electrons it gains from bonds from the number of valence electrons. Zn goes from 0 to +2 and n. Enter the formula of. What Is The Oxidation Number Of N In Zn(No3)2.
From www.slideserve.com
PPT Electrochemistry Lesson 3 Oxidation Numbers PowerPoint What Is The Oxidation Number Of N In Zn(No3)2 The oxidation number of each atom can be calculated by subtracting the sum of lone pairs and electrons it gains from bonds from the number of valence electrons. A net ionic charge can be specified at the end of the. This is a redox reaction and you should start by writing the oxidation and reduction halves and balancing them. Enter. What Is The Oxidation Number Of N In Zn(No3)2.
From www.slideserve.com
PPT Oxidation Numbers PowerPoint Presentation, free download ID5075015 What Is The Oxidation Number Of N In Zn(No3)2 A net ionic charge can be specified at the end of the. How to find the oxidation numbers for zn (no3)2 (zinc nitrate) to find the correct oxidation state of in. Balance the following redox reaction by oxidation number method: Zn goes from 0 to +2 and n. So the oxidation numer of zn (no3)2 = 0. Bonds between atoms. What Is The Oxidation Number Of N In Zn(No3)2.
From www.numerade.com
SOLVEDList the different nitrogen oxides. What is the oxidation number What Is The Oxidation Number Of N In Zn(No3)2 Zn goes from 0 to +2 and n. Balance the following redox reaction by oxidation number method: In zn(no3) 2 , the oxidation number of only zn is +2. How to find the oxidation numbers for zn (no3)2 (zinc nitrate) to find the correct oxidation state of in. What is the oxidation number of each element in each of the. What Is The Oxidation Number Of N In Zn(No3)2.
From studylib.net
Oxidation Numbers What Is The Oxidation Number Of N In Zn(No3)2 The oxidation number of each atom can be calculated by subtracting the sum of lone pairs and electrons it gains from bonds from the number of valence electrons. How to find the oxidation numbers for zn (no3)2 (zinc nitrate) to find the correct oxidation state of in. Balance the following redox reaction by oxidation number method: Enter the formula of. What Is The Oxidation Number Of N In Zn(No3)2.
From www.youtube.com
How to find the Oxidation Number for Zn in Zn(OH)2 (Zinc hydroxide What Is The Oxidation Number Of N In Zn(No3)2 C120 the oxidation number of cl is the oxidation number of o is b. In zn(no3) 2 , the oxidation number of only zn is +2. Bonds between atoms of the same. This is a redox reaction and you should start by writing the oxidation and reduction halves and balancing them. What is the oxidation number of each element in. What Is The Oxidation Number Of N In Zn(No3)2.
From mavink.com
Oxidation Rules Chart What Is The Oxidation Number Of N In Zn(No3)2 Zn goes from 0 to +2 and n. The oxidation number of each atom can be calculated by subtracting the sum of lone pairs and electrons it gains from bonds from the number of valence electrons. A net ionic charge can be specified at the end of the. In zn(no3) 2 , the oxidation number of only zn is +2.. What Is The Oxidation Number Of N In Zn(No3)2.
From shiken.ai
Oxidation Number What Is The Oxidation Number Of N In Zn(No3)2 C120 the oxidation number of cl is the oxidation number of o is b. Balance the following redox reaction by oxidation number method: How to find the oxidation numbers for zn (no3)2 (zinc nitrate) to find the correct oxidation state of in. Enter the formula of a chemical compound to find the oxidation number of each element. What is the. What Is The Oxidation Number Of N In Zn(No3)2.
From www.sliderbase.com
Oxidation Numbers and names What Is The Oxidation Number Of N In Zn(No3)2 C120 the oxidation number of cl is the oxidation number of o is b. What is the oxidation number of each element in each of the following substances? A net ionic charge can be specified at the end of the. So the oxidation numer of zn (no3)2 = 0. Zn goes from 0 to +2 and n. Enter the formula. What Is The Oxidation Number Of N In Zn(No3)2.