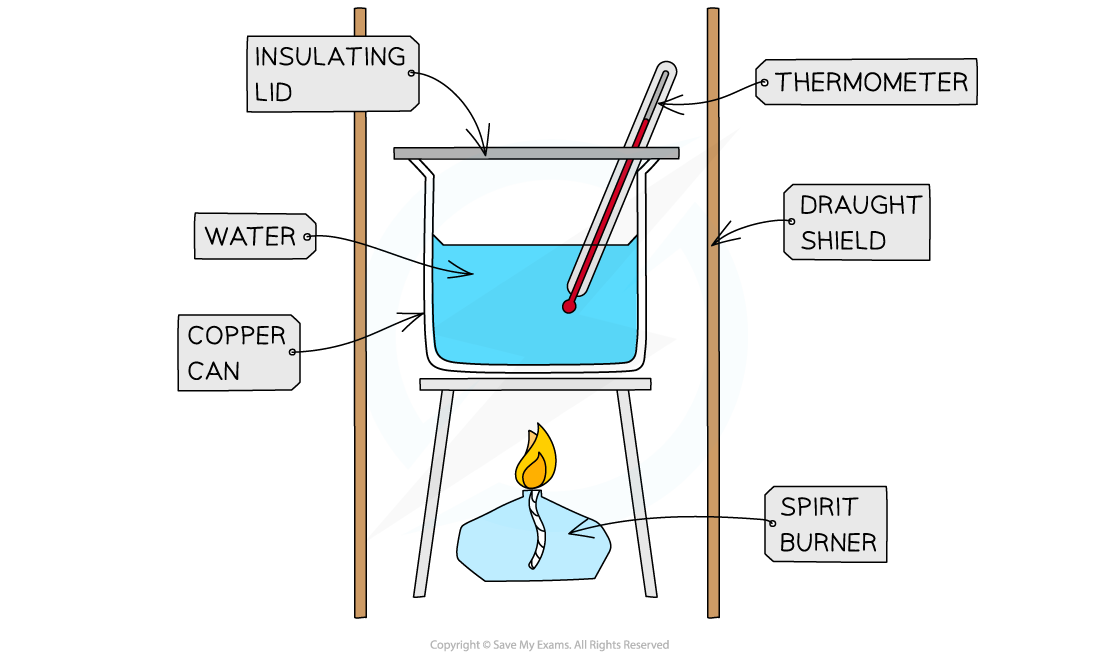
Calorimetry Specific Heat Capacity Of Metals Lab Report . To get a more accurate measure for specific heat of the metal, the heat capacity of the calorimeter can be determined. This document describes an experiment on determining the specific heat capacities of metals using calorimetry. Heat capacity by jamu alford with help from sari belzycki abstract using a styrofoam cup as a calorimeter the specific heat of water. In this experiment, you will determine the specific heat capacities of two different unknown metals by observing. Determination of the specific heat of a metal. Lansing community college general chemistry. Specific heat is the amount of energy, measured in joules, needed to raise the temperature of one gram of the substance one celsius degree. The major results for the three quantitative measurements include the average heat energy ( t)δ within the substances. 1) solving the heat equation for c,. The experiment involves measuring the temperature change of.
from www.hanlin.com
Lansing community college general chemistry. Specific heat is the amount of energy, measured in joules, needed to raise the temperature of one gram of the substance one celsius degree. Determination of the specific heat of a metal. Heat capacity by jamu alford with help from sari belzycki abstract using a styrofoam cup as a calorimeter the specific heat of water. The experiment involves measuring the temperature change of. The major results for the three quantitative measurements include the average heat energy ( t)δ within the substances. This document describes an experiment on determining the specific heat capacities of metals using calorimetry. 1) solving the heat equation for c,. In this experiment, you will determine the specific heat capacities of two different unknown metals by observing. To get a more accurate measure for specific heat of the metal, the heat capacity of the calorimeter can be determined.
IB DP Chemistry HL复习笔记5.1.4 Calorimetry Experiments翰林国际教育
Calorimetry Specific Heat Capacity Of Metals Lab Report Lansing community college general chemistry. This document describes an experiment on determining the specific heat capacities of metals using calorimetry. In this experiment, you will determine the specific heat capacities of two different unknown metals by observing. The experiment involves measuring the temperature change of. Lansing community college general chemistry. Specific heat is the amount of energy, measured in joules, needed to raise the temperature of one gram of the substance one celsius degree. The major results for the three quantitative measurements include the average heat energy ( t)δ within the substances. To get a more accurate measure for specific heat of the metal, the heat capacity of the calorimeter can be determined. Heat capacity by jamu alford with help from sari belzycki abstract using a styrofoam cup as a calorimeter the specific heat of water. Determination of the specific heat of a metal. 1) solving the heat equation for c,.
From www.studocu.com
Lab Report Specific Heat capacity of metals Background Chemists Calorimetry Specific Heat Capacity Of Metals Lab Report Heat capacity by jamu alford with help from sari belzycki abstract using a styrofoam cup as a calorimeter the specific heat of water. Lansing community college general chemistry. Determination of the specific heat of a metal. In this experiment, you will determine the specific heat capacities of two different unknown metals by observing. To get a more accurate measure for. Calorimetry Specific Heat Capacity Of Metals Lab Report.
From www.youtube.com
Principle of Calorimetry YouTube Calorimetry Specific Heat Capacity Of Metals Lab Report Heat capacity by jamu alford with help from sari belzycki abstract using a styrofoam cup as a calorimeter the specific heat of water. Determination of the specific heat of a metal. In this experiment, you will determine the specific heat capacities of two different unknown metals by observing. Lansing community college general chemistry. To get a more accurate measure for. Calorimetry Specific Heat Capacity Of Metals Lab Report.
From askfilo.com
Calorimetry Meaning, Specific heat capacities, Principle of heat capaci.. Calorimetry Specific Heat Capacity Of Metals Lab Report This document describes an experiment on determining the specific heat capacities of metals using calorimetry. Lansing community college general chemistry. In this experiment, you will determine the specific heat capacities of two different unknown metals by observing. Heat capacity by jamu alford with help from sari belzycki abstract using a styrofoam cup as a calorimeter the specific heat of water.. Calorimetry Specific Heat Capacity Of Metals Lab Report.
From www.scribd.com
lab 4 calorimetry lab Temperature Heat Capacity Calorimetry Specific Heat Capacity Of Metals Lab Report 1) solving the heat equation for c,. This document describes an experiment on determining the specific heat capacities of metals using calorimetry. Specific heat is the amount of energy, measured in joules, needed to raise the temperature of one gram of the substance one celsius degree. The experiment involves measuring the temperature change of. Lansing community college general chemistry. To. Calorimetry Specific Heat Capacity Of Metals Lab Report.
From www.docsity.com
Calorimetry lab report Study Guides, Projects, Research Chemistry Calorimetry Specific Heat Capacity Of Metals Lab Report 1) solving the heat equation for c,. Heat capacity by jamu alford with help from sari belzycki abstract using a styrofoam cup as a calorimeter the specific heat of water. Determination of the specific heat of a metal. To get a more accurate measure for specific heat of the metal, the heat capacity of the calorimeter can be determined. The. Calorimetry Specific Heat Capacity Of Metals Lab Report.
From fyogshfpe.blob.core.windows.net
Lab Calorimetry And Specific Heat Data Table at Russell Kelly blog Calorimetry Specific Heat Capacity Of Metals Lab Report This document describes an experiment on determining the specific heat capacities of metals using calorimetry. The major results for the three quantitative measurements include the average heat energy ( t)δ within the substances. The experiment involves measuring the temperature change of. In this experiment, you will determine the specific heat capacities of two different unknown metals by observing. Lansing community. Calorimetry Specific Heat Capacity Of Metals Lab Report.
From janiyahabbgates.blogspot.com
Calorimetry Specific Heat Capacity of Metals Lab Report JaniyahabbGates Calorimetry Specific Heat Capacity Of Metals Lab Report The major results for the three quantitative measurements include the average heat energy ( t)δ within the substances. This document describes an experiment on determining the specific heat capacities of metals using calorimetry. 1) solving the heat equation for c,. In this experiment, you will determine the specific heat capacities of two different unknown metals by observing. Lansing community college. Calorimetry Specific Heat Capacity Of Metals Lab Report.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Calorimetry Specific Heat Capacity Of Metals Lab Report Heat capacity by jamu alford with help from sari belzycki abstract using a styrofoam cup as a calorimeter the specific heat of water. Specific heat is the amount of energy, measured in joules, needed to raise the temperature of one gram of the substance one celsius degree. This document describes an experiment on determining the specific heat capacities of metals. Calorimetry Specific Heat Capacity Of Metals Lab Report.
From vittaeducation.com
PASCO Scientific Practical Specific Heat Capacity VITTA Education Calorimetry Specific Heat Capacity Of Metals Lab Report This document describes an experiment on determining the specific heat capacities of metals using calorimetry. To get a more accurate measure for specific heat of the metal, the heat capacity of the calorimeter can be determined. 1) solving the heat equation for c,. The major results for the three quantitative measurements include the average heat energy ( t)δ within the. Calorimetry Specific Heat Capacity Of Metals Lab Report.
From www.chegg.com
Calorimetry Specific Heat Capacity of a Metal Calorimetry Specific Heat Capacity Of Metals Lab Report This document describes an experiment on determining the specific heat capacities of metals using calorimetry. To get a more accurate measure for specific heat of the metal, the heat capacity of the calorimeter can be determined. The major results for the three quantitative measurements include the average heat energy ( t)δ within the substances. 1) solving the heat equation for. Calorimetry Specific Heat Capacity Of Metals Lab Report.
From www.edrawmax.com
Calorimetry Lab Report EdrawMax Template Calorimetry Specific Heat Capacity Of Metals Lab Report 1) solving the heat equation for c,. Lansing community college general chemistry. The major results for the three quantitative measurements include the average heat energy ( t)δ within the substances. The experiment involves measuring the temperature change of. This document describes an experiment on determining the specific heat capacities of metals using calorimetry. Determination of the specific heat of a. Calorimetry Specific Heat Capacity Of Metals Lab Report.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Calorimetry Specific Heat Capacity Of Metals Lab Report This document describes an experiment on determining the specific heat capacities of metals using calorimetry. 1) solving the heat equation for c,. Specific heat is the amount of energy, measured in joules, needed to raise the temperature of one gram of the substance one celsius degree. Lansing community college general chemistry. Heat capacity by jamu alford with help from sari. Calorimetry Specific Heat Capacity Of Metals Lab Report.
From www.chegg.com
Solved Experiment 21 Report Sheet Calorimetry Date A. Calorimetry Specific Heat Capacity Of Metals Lab Report Lansing community college general chemistry. Heat capacity by jamu alford with help from sari belzycki abstract using a styrofoam cup as a calorimeter the specific heat of water. The experiment involves measuring the temperature change of. Determination of the specific heat of a metal. 1) solving the heat equation for c,. In this experiment, you will determine the specific heat. Calorimetry Specific Heat Capacity Of Metals Lab Report.
From www.linstitute.net
Edexcel A Level Chemistry复习笔记4.1.4 Determining Enthalpy Change of Calorimetry Specific Heat Capacity Of Metals Lab Report Lansing community college general chemistry. 1) solving the heat equation for c,. Determination of the specific heat of a metal. Heat capacity by jamu alford with help from sari belzycki abstract using a styrofoam cup as a calorimeter the specific heat of water. The major results for the three quantitative measurements include the average heat energy ( t)δ within the. Calorimetry Specific Heat Capacity Of Metals Lab Report.
From janiyahabbgates.blogspot.com
Calorimetry Specific Heat Capacity of Metals Lab Report JaniyahabbGates Calorimetry Specific Heat Capacity Of Metals Lab Report Determination of the specific heat of a metal. Lansing community college general chemistry. In this experiment, you will determine the specific heat capacities of two different unknown metals by observing. The experiment involves measuring the temperature change of. Specific heat is the amount of energy, measured in joules, needed to raise the temperature of one gram of the substance one. Calorimetry Specific Heat Capacity Of Metals Lab Report.
From janiyahabbgates.blogspot.com
Calorimetry Specific Heat Capacity of Metals Lab Report JaniyahabbGates Calorimetry Specific Heat Capacity Of Metals Lab Report 1) solving the heat equation for c,. In this experiment, you will determine the specific heat capacities of two different unknown metals by observing. Lansing community college general chemistry. To get a more accurate measure for specific heat of the metal, the heat capacity of the calorimeter can be determined. The experiment involves measuring the temperature change of. Determination of. Calorimetry Specific Heat Capacity Of Metals Lab Report.
From mavink.com
Specific Heat Capacity Diagram Calorimetry Specific Heat Capacity Of Metals Lab Report Specific heat is the amount of energy, measured in joules, needed to raise the temperature of one gram of the substance one celsius degree. In this experiment, you will determine the specific heat capacities of two different unknown metals by observing. The experiment involves measuring the temperature change of. Lansing community college general chemistry. 1) solving the heat equation for. Calorimetry Specific Heat Capacity Of Metals Lab Report.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Calorimetry Specific Heat Capacity Of Metals Lab Report Heat capacity by jamu alford with help from sari belzycki abstract using a styrofoam cup as a calorimeter the specific heat of water. This document describes an experiment on determining the specific heat capacities of metals using calorimetry. 1) solving the heat equation for c,. Specific heat is the amount of energy, measured in joules, needed to raise the temperature. Calorimetry Specific Heat Capacity Of Metals Lab Report.
From ar.inspiredpencil.com
Specific Heat Lab Calorimeter Calorimetry Specific Heat Capacity Of Metals Lab Report Heat capacity by jamu alford with help from sari belzycki abstract using a styrofoam cup as a calorimeter the specific heat of water. 1) solving the heat equation for c,. Specific heat is the amount of energy, measured in joules, needed to raise the temperature of one gram of the substance one celsius degree. The major results for the three. Calorimetry Specific Heat Capacity Of Metals Lab Report.
From www.scribd.com
Calorimetry Lab Heat Capacity Heat Calorimetry Specific Heat Capacity Of Metals Lab Report The major results for the three quantitative measurements include the average heat energy ( t)δ within the substances. To get a more accurate measure for specific heat of the metal, the heat capacity of the calorimeter can be determined. Heat capacity by jamu alford with help from sari belzycki abstract using a styrofoam cup as a calorimeter the specific heat. Calorimetry Specific Heat Capacity Of Metals Lab Report.
From www.chegg.com
Solved Table 2a. Specific Heat Capacity of Metal Sample 1 Calorimetry Specific Heat Capacity Of Metals Lab Report Heat capacity by jamu alford with help from sari belzycki abstract using a styrofoam cup as a calorimeter the specific heat of water. Determination of the specific heat of a metal. This document describes an experiment on determining the specific heat capacities of metals using calorimetry. The experiment involves measuring the temperature change of. To get a more accurate measure. Calorimetry Specific Heat Capacity Of Metals Lab Report.
From www.researchgate.net
(PDF) “Greening” a Familiar General Chemistry Experiment Coffee Cup Calorimetry Specific Heat Capacity Of Metals Lab Report 1) solving the heat equation for c,. This document describes an experiment on determining the specific heat capacities of metals using calorimetry. The experiment involves measuring the temperature change of. The major results for the three quantitative measurements include the average heat energy ( t)δ within the substances. Specific heat is the amount of energy, measured in joules, needed to. Calorimetry Specific Heat Capacity Of Metals Lab Report.
From www.linstitute.net
AQA A Level Physics复习笔记6.4.3 Specific Heat Capacity翰林国际教育 Calorimetry Specific Heat Capacity Of Metals Lab Report 1) solving the heat equation for c,. In this experiment, you will determine the specific heat capacities of two different unknown metals by observing. The experiment involves measuring the temperature change of. To get a more accurate measure for specific heat of the metal, the heat capacity of the calorimeter can be determined. The major results for the three quantitative. Calorimetry Specific Heat Capacity Of Metals Lab Report.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Calorimetry Specific Heat Capacity Of Metals Lab Report To get a more accurate measure for specific heat of the metal, the heat capacity of the calorimeter can be determined. In this experiment, you will determine the specific heat capacities of two different unknown metals by observing. Lansing community college general chemistry. This document describes an experiment on determining the specific heat capacities of metals using calorimetry. Specific heat. Calorimetry Specific Heat Capacity Of Metals Lab Report.
From www.studocu.com
Calorimetry Lab Report 04/13/ CHEM 1300 Calorimetry PreLaboratory Calorimetry Specific Heat Capacity Of Metals Lab Report Heat capacity by jamu alford with help from sari belzycki abstract using a styrofoam cup as a calorimeter the specific heat of water. 1) solving the heat equation for c,. The major results for the three quantitative measurements include the average heat energy ( t)δ within the substances. Determination of the specific heat of a metal. Specific heat is the. Calorimetry Specific Heat Capacity Of Metals Lab Report.
From www.hanlin.com
IB DP Chemistry HL复习笔记5.1.4 Calorimetry Experiments翰林国际教育 Calorimetry Specific Heat Capacity Of Metals Lab Report In this experiment, you will determine the specific heat capacities of two different unknown metals by observing. 1) solving the heat equation for c,. The major results for the three quantitative measurements include the average heat energy ( t)δ within the substances. This document describes an experiment on determining the specific heat capacities of metals using calorimetry. Heat capacity by. Calorimetry Specific Heat Capacity Of Metals Lab Report.
From webapi.bu.edu
💣 Specific heat of a metal lab conclusion. Specific Heat of Metal Lab Calorimetry Specific Heat Capacity Of Metals Lab Report Heat capacity by jamu alford with help from sari belzycki abstract using a styrofoam cup as a calorimeter the specific heat of water. To get a more accurate measure for specific heat of the metal, the heat capacity of the calorimeter can be determined. The experiment involves measuring the temperature change of. Specific heat is the amount of energy, measured. Calorimetry Specific Heat Capacity Of Metals Lab Report.
From brainly.com
calorimetry and specific heat lab report can someone please write this Calorimetry Specific Heat Capacity Of Metals Lab Report Determination of the specific heat of a metal. Specific heat is the amount of energy, measured in joules, needed to raise the temperature of one gram of the substance one celsius degree. Lansing community college general chemistry. In this experiment, you will determine the specific heat capacities of two different unknown metals by observing. Heat capacity by jamu alford with. Calorimetry Specific Heat Capacity Of Metals Lab Report.
From www.chegg.com
Solved Specific Heat Capacity of Metals Peter Jeschofnig, Calorimetry Specific Heat Capacity Of Metals Lab Report The major results for the three quantitative measurements include the average heat energy ( t)δ within the substances. This document describes an experiment on determining the specific heat capacities of metals using calorimetry. 1) solving the heat equation for c,. Lansing community college general chemistry. To get a more accurate measure for specific heat of the metal, the heat capacity. Calorimetry Specific Heat Capacity Of Metals Lab Report.
From wisc.pb.unizin.org
5.2 Calorimetry Chemistry Calorimetry Specific Heat Capacity Of Metals Lab Report Determination of the specific heat of a metal. 1) solving the heat equation for c,. The major results for the three quantitative measurements include the average heat energy ( t)δ within the substances. Lansing community college general chemistry. This document describes an experiment on determining the specific heat capacities of metals using calorimetry. Specific heat is the amount of energy,. Calorimetry Specific Heat Capacity Of Metals Lab Report.
From www.chegg.com
Laboratory 9 CALORIMETRY SPECIFIC HEAT OF A METAL Calorimetry Specific Heat Capacity Of Metals Lab Report Lansing community college general chemistry. The experiment involves measuring the temperature change of. The major results for the three quantitative measurements include the average heat energy ( t)δ within the substances. Heat capacity by jamu alford with help from sari belzycki abstract using a styrofoam cup as a calorimeter the specific heat of water. This document describes an experiment on. Calorimetry Specific Heat Capacity Of Metals Lab Report.
From ar.inspiredpencil.com
Heat Definition Calorimetry Specific Heat Capacity Of Metals Lab Report The major results for the three quantitative measurements include the average heat energy ( t)δ within the substances. 1) solving the heat equation for c,. The experiment involves measuring the temperature change of. Lansing community college general chemistry. In this experiment, you will determine the specific heat capacities of two different unknown metals by observing. To get a more accurate. Calorimetry Specific Heat Capacity Of Metals Lab Report.
From users.highland.edu
Calorimetry Calorimetry Specific Heat Capacity Of Metals Lab Report 1) solving the heat equation for c,. Heat capacity by jamu alford with help from sari belzycki abstract using a styrofoam cup as a calorimeter the specific heat of water. Specific heat is the amount of energy, measured in joules, needed to raise the temperature of one gram of the substance one celsius degree. Lansing community college general chemistry. To. Calorimetry Specific Heat Capacity Of Metals Lab Report.
From www.scribd.com
Lab Report 1 Calorimetry Specific Heat Capacities of Metals PDF Calorimetry Specific Heat Capacity Of Metals Lab Report Heat capacity by jamu alford with help from sari belzycki abstract using a styrofoam cup as a calorimeter the specific heat of water. To get a more accurate measure for specific heat of the metal, the heat capacity of the calorimeter can be determined. Lansing community college general chemistry. Determination of the specific heat of a metal. 1) solving the. Calorimetry Specific Heat Capacity Of Metals Lab Report.
From www.chegg.com
Solved Report on Laboratory Experiment "Specific Heat of a Calorimetry Specific Heat Capacity Of Metals Lab Report Specific heat is the amount of energy, measured in joules, needed to raise the temperature of one gram of the substance one celsius degree. In this experiment, you will determine the specific heat capacities of two different unknown metals by observing. Determination of the specific heat of a metal. Lansing community college general chemistry. Heat capacity by jamu alford with. Calorimetry Specific Heat Capacity Of Metals Lab Report.