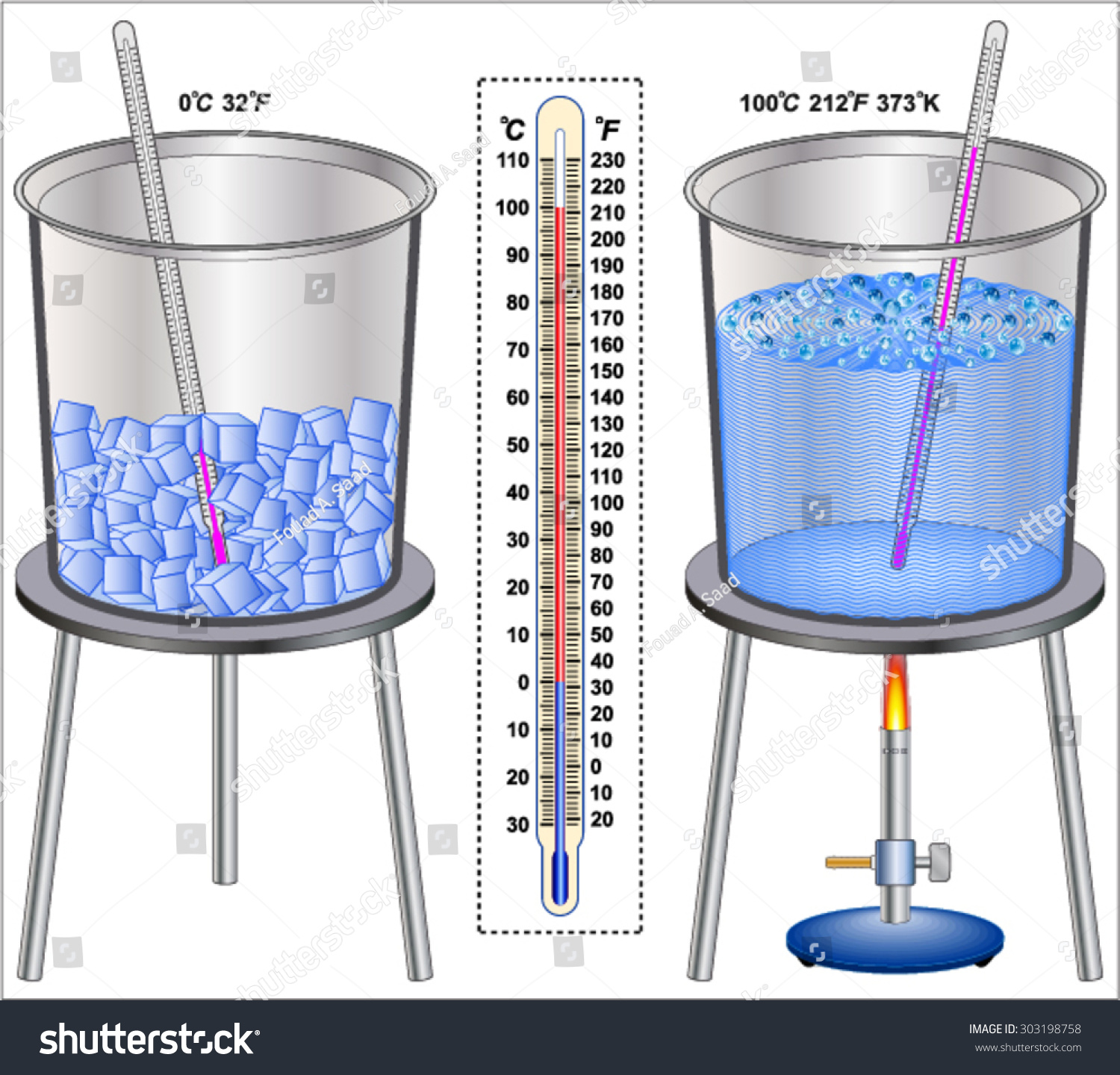
What Temperature Is Boiling . Find out the boiling point of water at different locations and see how to boil. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapor of the liquid;. The boiling point of water is 100 °c or 212 °f but is lower with the decreased atmospheric pressure found at higher altitudes. Boiling point is the temperature that a liquid will change phase into a gas. Boiling occurs when the vapour pressure of a liquid is equal to the atmospheric pressure of the gas outside of. The boiling point of a liquid depends on temperature,. For example, for water, the boiling point is 100ºc at a pressure of 1 atm. Learn how atmospheric pressure, elevation, and impurities affect the boiling point of water. If you want a quick and simple answer, you can say that the boiling point of water is 100 °c or 212 °f at 1 atmosphere of pressure.
from www.shutterstock.com
Boiling point is the temperature that a liquid will change phase into a gas. Find out the boiling point of water at different locations and see how to boil. Boiling occurs when the vapour pressure of a liquid is equal to the atmospheric pressure of the gas outside of. The boiling point of water is 100 °c or 212 °f but is lower with the decreased atmospheric pressure found at higher altitudes. If you want a quick and simple answer, you can say that the boiling point of water is 100 °c or 212 °f at 1 atmosphere of pressure. Learn how atmospheric pressure, elevation, and impurities affect the boiling point of water. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapor of the liquid;. The boiling point of a liquid depends on temperature,. For example, for water, the boiling point is 100ºc at a pressure of 1 atm.
Temperature Ice And Boiling Water Stock Vector 303198758 Shutterstock
What Temperature Is Boiling For example, for water, the boiling point is 100ºc at a pressure of 1 atm. Boiling point is the temperature that a liquid will change phase into a gas. If you want a quick and simple answer, you can say that the boiling point of water is 100 °c or 212 °f at 1 atmosphere of pressure. Learn how atmospheric pressure, elevation, and impurities affect the boiling point of water. For example, for water, the boiling point is 100ºc at a pressure of 1 atm. The boiling point of water is 100 °c or 212 °f but is lower with the decreased atmospheric pressure found at higher altitudes. Boiling occurs when the vapour pressure of a liquid is equal to the atmospheric pressure of the gas outside of. The boiling point of a liquid depends on temperature,. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapor of the liquid;. Find out the boiling point of water at different locations and see how to boil.
From www.yiannislucacos.gr
How to boil Boiling as a basic cooking method Yiannis Lucacos What Temperature Is Boiling Learn how atmospheric pressure, elevation, and impurities affect the boiling point of water. Boiling occurs when the vapour pressure of a liquid is equal to the atmospheric pressure of the gas outside of. If you want a quick and simple answer, you can say that the boiling point of water is 100 °c or 212 °f at 1 atmosphere of. What Temperature Is Boiling.
From sciencenotes.org
Boiling Point of Water What Temperature Does Water Boil? What Temperature Is Boiling Find out the boiling point of water at different locations and see how to boil. Learn how atmospheric pressure, elevation, and impurities affect the boiling point of water. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapor of the liquid;. Boiling occurs when the vapour pressure. What Temperature Is Boiling.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps What Temperature Is Boiling For example, for water, the boiling point is 100ºc at a pressure of 1 atm. Learn how atmospheric pressure, elevation, and impurities affect the boiling point of water. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapor of the liquid;. The boiling point of a liquid. What Temperature Is Boiling.
From www.compoundchem.com
What Temperature Does Water Boil At? Boiling Point & Elevation What Temperature Is Boiling Boiling occurs when the vapour pressure of a liquid is equal to the atmospheric pressure of the gas outside of. The boiling point of a liquid depends on temperature,. For example, for water, the boiling point is 100ºc at a pressure of 1 atm. If you want a quick and simple answer, you can say that the boiling point of. What Temperature Is Boiling.
From www.jessicagavin.com
Boiling (MoistHeat Cooking Method) Jessica Gavin What Temperature Is Boiling Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapor of the liquid;. For example, for water, the boiling point is 100ºc at a pressure of 1 atm. If you want a quick and simple answer, you can say that the boiling point of water is 100. What Temperature Is Boiling.
From www.pinterest.com
Mastering Boiling Water Tips for Cooking at Altitude What Temperature Is Boiling Boiling occurs when the vapour pressure of a liquid is equal to the atmospheric pressure of the gas outside of. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapor of the liquid;. Boiling point is the temperature that a liquid will change phase into a gas.. What Temperature Is Boiling.
From www.gettyimages.ie
Freezing And Boiling Points In Celsius And Fahrenheit HighRes Vector What Temperature Is Boiling Learn how atmospheric pressure, elevation, and impurities affect the boiling point of water. For example, for water, the boiling point is 100ºc at a pressure of 1 atm. Boiling occurs when the vapour pressure of a liquid is equal to the atmospheric pressure of the gas outside of. The boiling point of water is 100 °c or 212 °f but. What Temperature Is Boiling.
From www.chefstemp.com
How to calibrate a thermometer in boiling water ChefsTemp What Temperature Is Boiling Boiling occurs when the vapour pressure of a liquid is equal to the atmospheric pressure of the gas outside of. Find out the boiling point of water at different locations and see how to boil. The boiling point of water is 100 °c or 212 °f but is lower with the decreased atmospheric pressure found at higher altitudes. Boiling point,. What Temperature Is Boiling.
From hikingmastery.com
Does Boiling Water Purify It Basic Facts and Useful What Temperature Is Boiling The boiling point of water is 100 °c or 212 °f but is lower with the decreased atmospheric pressure found at higher altitudes. The boiling point of a liquid depends on temperature,. Learn how atmospheric pressure, elevation, and impurities affect the boiling point of water. Find out the boiling point of water at different locations and see how to boil.. What Temperature Is Boiling.
From blog.thermoworks.com
Thermal Secrets to Boiling Point Calibration ThermoWorks What Temperature Is Boiling Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapor of the liquid;. If you want a quick and simple answer, you can say that the boiling point of water is 100 °c or 212 °f at 1 atmosphere of pressure. Find out the boiling point of. What Temperature Is Boiling.
From labbyag.es
Water Boiling Temperature Pressure Chart Labb by AG What Temperature Is Boiling The boiling point of water is 100 °c or 212 °f but is lower with the decreased atmospheric pressure found at higher altitudes. If you want a quick and simple answer, you can say that the boiling point of water is 100 °c or 212 °f at 1 atmosphere of pressure. The boiling point of a liquid depends on temperature,.. What Temperature Is Boiling.
From www.boiler-planning.com
Boiling pressure and temperature Bosch Steam boiler planning What Temperature Is Boiling Find out the boiling point of water at different locations and see how to boil. Boiling point is the temperature that a liquid will change phase into a gas. Learn how atmospheric pressure, elevation, and impurities affect the boiling point of water. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the. What Temperature Is Boiling.
From sciencenotes.org
How to Boil Water at Room Temperature What Temperature Is Boiling Find out the boiling point of water at different locations and see how to boil. Boiling point is the temperature that a liquid will change phase into a gas. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapor of the liquid;. Boiling occurs when the vapour. What Temperature Is Boiling.
From kenkidryer.com
Saturation temperature (boiling point) KENKI DRYER What Temperature Is Boiling The boiling point of water is 100 °c or 212 °f but is lower with the decreased atmospheric pressure found at higher altitudes. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapor of the liquid;. Learn how atmospheric pressure, elevation, and impurities affect the boiling point. What Temperature Is Boiling.
From studylib.net
Boiling Point = Temperature at which a liquid turns into a gas What Temperature Is Boiling Boiling occurs when the vapour pressure of a liquid is equal to the atmospheric pressure of the gas outside of. The boiling point of a liquid depends on temperature,. Find out the boiling point of water at different locations and see how to boil. The boiling point of water is 100 °c or 212 °f but is lower with the. What Temperature Is Boiling.
From favpng.com
Boiling Point Melting Point Heat Temperature Chemistry, PNG What Temperature Is Boiling For example, for water, the boiling point is 100ºc at a pressure of 1 atm. Learn how atmospheric pressure, elevation, and impurities affect the boiling point of water. If you want a quick and simple answer, you can say that the boiling point of water is 100 °c or 212 °f at 1 atmosphere of pressure. The boiling point of. What Temperature Is Boiling.
From www.nsta.org
Boiling Time and Temperature NSTA What Temperature Is Boiling The boiling point of a liquid depends on temperature,. For example, for water, the boiling point is 100ºc at a pressure of 1 atm. If you want a quick and simple answer, you can say that the boiling point of water is 100 °c or 212 °f at 1 atmosphere of pressure. Boiling occurs when the vapour pressure of a. What Temperature Is Boiling.
From www.dreamstime.com
Boiling and Evaporation, Freezing and Melting Points of Water Stock What Temperature Is Boiling Find out the boiling point of water at different locations and see how to boil. Boiling occurs when the vapour pressure of a liquid is equal to the atmospheric pressure of the gas outside of. If you want a quick and simple answer, you can say that the boiling point of water is 100 °c or 212 °f at 1. What Temperature Is Boiling.
From sciencenotes.org
Boiling Point Definition, Temperature, and Examples What Temperature Is Boiling For example, for water, the boiling point is 100ºc at a pressure of 1 atm. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapor of the liquid;. The boiling point of a liquid depends on temperature,. Learn how atmospheric pressure, elevation, and impurities affect the boiling. What Temperature Is Boiling.
From www.sciencephoto.com
temperature of boiling oil Stock Image C029/7191 Science Photo What Temperature Is Boiling The boiling point of a liquid depends on temperature,. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapor of the liquid;. Find out the boiling point of water at different locations and see how to boil. If you want a quick and simple answer, you can. What Temperature Is Boiling.
From howchimp.com
What Is the Boiling Point of Water in Kelvin, Celsius, and Fahrenheit What Temperature Is Boiling Boiling point is the temperature that a liquid will change phase into a gas. The boiling point of water is 100 °c or 212 °f but is lower with the decreased atmospheric pressure found at higher altitudes. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapor. What Temperature Is Boiling.
From www.momswhothink.com
Simmer vs. Boil How to Tell the Differences (With Temperatures) What Temperature Is Boiling The boiling point of water is 100 °c or 212 °f but is lower with the decreased atmospheric pressure found at higher altitudes. For example, for water, the boiling point is 100ºc at a pressure of 1 atm. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the. What Temperature Is Boiling.
From freerangestock.com
Free Stock Photo of Thermometer Hot Represents Temperature Indicator What Temperature Is Boiling Learn how atmospheric pressure, elevation, and impurities affect the boiling point of water. The boiling point of a liquid depends on temperature,. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapor of the liquid;. If you want a quick and simple answer, you can say that. What Temperature Is Boiling.
From worldnewlive.com
At What Temperature Did The Water Start To Boil? Mastery Wiki What Temperature Is Boiling Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapor of the liquid;. The boiling point of a liquid depends on temperature,. For example, for water, the boiling point is 100ºc at a pressure of 1 atm. The boiling point of water is 100 °c or 212. What Temperature Is Boiling.
From www.pinterest.com
Boiling Point Easy Science What Temperature Is Boiling Boiling point is the temperature that a liquid will change phase into a gas. Learn how atmospheric pressure, elevation, and impurities affect the boiling point of water. The boiling point of a liquid depends on temperature,. Find out the boiling point of water at different locations and see how to boil. Boiling point, temperature at which the pressure exerted by. What Temperature Is Boiling.
From www.researchgate.net
The vapor pressure of ethanol vs. the normal boilingpoint temperature What Temperature Is Boiling The boiling point of a liquid depends on temperature,. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapor of the liquid;. For example, for water, the boiling point is 100ºc at a pressure of 1 atm. Boiling occurs when the vapour pressure of a liquid is. What Temperature Is Boiling.
From sciencenotes.org
How to Boil Water at Room Temperature What Temperature Is Boiling Learn how atmospheric pressure, elevation, and impurities affect the boiling point of water. Boiling point is the temperature that a liquid will change phase into a gas. Find out the boiling point of water at different locations and see how to boil. The boiling point of water is 100 °c or 212 °f but is lower with the decreased atmospheric. What Temperature Is Boiling.
From socratic.org
What is the relation between critical temperature and boiling point or What Temperature Is Boiling Boiling point is the temperature that a liquid will change phase into a gas. If you want a quick and simple answer, you can say that the boiling point of water is 100 °c or 212 °f at 1 atmosphere of pressure. The boiling point of water is 100 °c or 212 °f but is lower with the decreased atmospheric. What Temperature Is Boiling.
From www.shutterstock.com
Temperature Ice And Boiling Water Stock Vector 303198758 Shutterstock What Temperature Is Boiling Find out the boiling point of water at different locations and see how to boil. Boiling point is the temperature that a liquid will change phase into a gas. If you want a quick and simple answer, you can say that the boiling point of water is 100 °c or 212 °f at 1 atmosphere of pressure. Learn how atmospheric. What Temperature Is Boiling.
From www.rmets.org
The highs, lows and feels of temperature Royal Meteorological Society What Temperature Is Boiling Find out the boiling point of water at different locations and see how to boil. The boiling point of water is 100 °c or 212 °f but is lower with the decreased atmospheric pressure found at higher altitudes. Boiling point is the temperature that a liquid will change phase into a gas. If you want a quick and simple answer,. What Temperature Is Boiling.
From apollo.lsc.vsc.edu
Saturation Vapor Pressure and the Boiling Point What Temperature Is Boiling Learn how atmospheric pressure, elevation, and impurities affect the boiling point of water. Boiling occurs when the vapour pressure of a liquid is equal to the atmospheric pressure of the gas outside of. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapor of the liquid;. Boiling. What Temperature Is Boiling.
From www.slideserve.com
PPT Freezing/Melting and Boiling Points PowerPoint Presentation, free What Temperature Is Boiling For example, for water, the boiling point is 100ºc at a pressure of 1 atm. Boiling occurs when the vapour pressure of a liquid is equal to the atmospheric pressure of the gas outside of. The boiling point of a liquid depends on temperature,. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled. What Temperature Is Boiling.
From sciencenotes.org
Does Boiling Water Keep Getting Hotter? What Temperature Is Boiling Boiling point is the temperature that a liquid will change phase into a gas. Boiling occurs when the vapour pressure of a liquid is equal to the atmospheric pressure of the gas outside of. The boiling point of water is 100 °c or 212 °f but is lower with the decreased atmospheric pressure found at higher altitudes. If you want. What Temperature Is Boiling.
From www.sciencephoto.com
temperature of boiling oil Stock Image C029/7165 Science Photo What Temperature Is Boiling Learn how atmospheric pressure, elevation, and impurities affect the boiling point of water. Find out the boiling point of water at different locations and see how to boil. The boiling point of a liquid depends on temperature,. For example, for water, the boiling point is 100ºc at a pressure of 1 atm. Boiling occurs when the vapour pressure of a. What Temperature Is Boiling.
From blog.sciencescore.com
What temperature does water boil? What Temperature Is Boiling Boiling point is the temperature that a liquid will change phase into a gas. Find out the boiling point of water at different locations and see how to boil. Boiling occurs when the vapour pressure of a liquid is equal to the atmospheric pressure of the gas outside of. Learn how atmospheric pressure, elevation, and impurities affect the boiling point. What Temperature Is Boiling.