Why Is An Ice Bath Used For Crystallization . Use a lower temperature bath to try and encourage crystal formation. The product may be washed with ice. If the compound has dissolved in boiling solvent (either 0.25 or 0.5 ml), cool the tube in cold water to see if crystallization occurs. You may use a spatula to assist in transferring the crystals to the funnel, then wash the phthalic acid in the. If no crystals form even after the solution has been cooled in an ice bath, take a fire polished stirring rod and etch (scratch) the glass of your. Crystallization can be a slow process, and impatience can lead to low recovery. Place a disk of filter paper in the funnel and collect the crystals by vacuum filtration. The difference in crystal lattice energy between pure and impure solids is marginal, so a solution must be cooled slowly to allow for differentiation. Recrystallisation may be done in a multitude of ways, depending on your particular situation; If a hot solution is plunged immediately into an.
from informacionpublica.svet.gob.gt
Crystallization can be a slow process, and impatience can lead to low recovery. If the compound has dissolved in boiling solvent (either 0.25 or 0.5 ml), cool the tube in cold water to see if crystallization occurs. The product may be washed with ice. Place a disk of filter paper in the funnel and collect the crystals by vacuum filtration. You may use a spatula to assist in transferring the crystals to the funnel, then wash the phthalic acid in the. If a hot solution is plunged immediately into an. Use a lower temperature bath to try and encourage crystal formation. If no crystals form even after the solution has been cooled in an ice bath, take a fire polished stirring rod and etch (scratch) the glass of your. Recrystallisation may be done in a multitude of ways, depending on your particular situation; The difference in crystal lattice energy between pure and impure solids is marginal, so a solution must be cooled slowly to allow for differentiation.
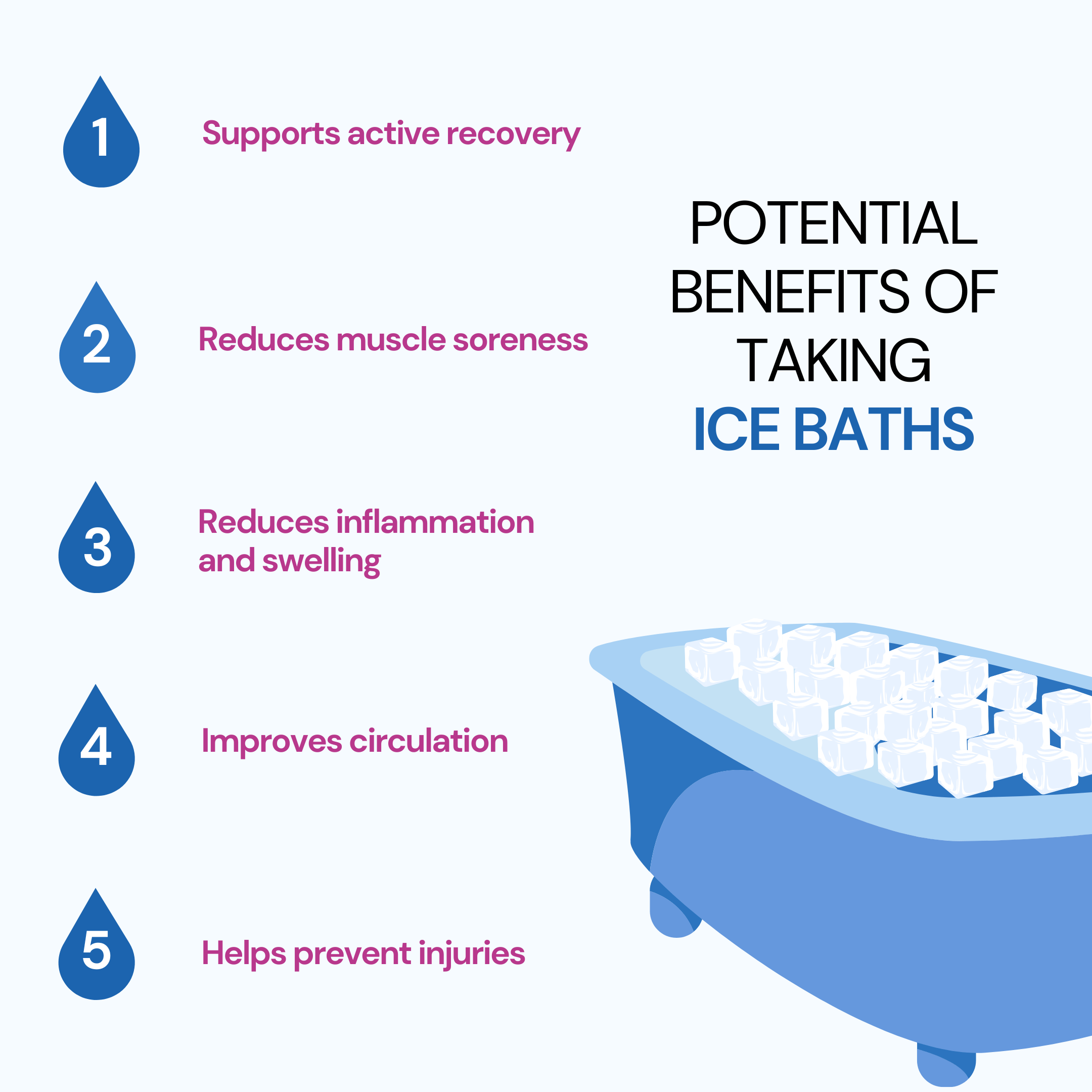
Benefits Of Ice Baths For Muscles informacionpublica.svet.gob.gt
Why Is An Ice Bath Used For Crystallization You may use a spatula to assist in transferring the crystals to the funnel, then wash the phthalic acid in the. Place a disk of filter paper in the funnel and collect the crystals by vacuum filtration. Use a lower temperature bath to try and encourage crystal formation. If the compound has dissolved in boiling solvent (either 0.25 or 0.5 ml), cool the tube in cold water to see if crystallization occurs. Recrystallisation may be done in a multitude of ways, depending on your particular situation; The difference in crystal lattice energy between pure and impure solids is marginal, so a solution must be cooled slowly to allow for differentiation. If no crystals form even after the solution has been cooled in an ice bath, take a fire polished stirring rod and etch (scratch) the glass of your. Crystallization can be a slow process, and impatience can lead to low recovery. If a hot solution is plunged immediately into an. The product may be washed with ice. You may use a spatula to assist in transferring the crystals to the funnel, then wash the phthalic acid in the.
From spmchemistry.blog.onlinetuition.com.my
Three States of Matter Structured Question 4 SPM Chemistry Why Is An Ice Bath Used For Crystallization Recrystallisation may be done in a multitude of ways, depending on your particular situation; You may use a spatula to assist in transferring the crystals to the funnel, then wash the phthalic acid in the. The difference in crystal lattice energy between pure and impure solids is marginal, so a solution must be cooled slowly to allow for differentiation. The. Why Is An Ice Bath Used For Crystallization.
From byjus.com
Crystallization Definition, Process, Separation Technique, FAQs Why Is An Ice Bath Used For Crystallization If no crystals form even after the solution has been cooled in an ice bath, take a fire polished stirring rod and etch (scratch) the glass of your. You may use a spatula to assist in transferring the crystals to the funnel, then wash the phthalic acid in the. The difference in crystal lattice energy between pure and impure solids. Why Is An Ice Bath Used For Crystallization.
From www.chegg.com
Solved 5. Why should the recrystallization mixture be cooled Why Is An Ice Bath Used For Crystallization The difference in crystal lattice energy between pure and impure solids is marginal, so a solution must be cooled slowly to allow for differentiation. Crystallization can be a slow process, and impatience can lead to low recovery. If a hot solution is plunged immediately into an. If the compound has dissolved in boiling solvent (either 0.25 or 0.5 ml), cool. Why Is An Ice Bath Used For Crystallization.
From www.myglobalviewpoint.com
Ice Bath Benefits Research + Tips for Ice Bathing at Home Why Is An Ice Bath Used For Crystallization Place a disk of filter paper in the funnel and collect the crystals by vacuum filtration. Use a lower temperature bath to try and encourage crystal formation. The product may be washed with ice. If the compound has dissolved in boiling solvent (either 0.25 or 0.5 ml), cool the tube in cold water to see if crystallization occurs. Crystallization can. Why Is An Ice Bath Used For Crystallization.
From rebathal.com
Why Ice Bath After Sous Vide rebathal Why Is An Ice Bath Used For Crystallization You may use a spatula to assist in transferring the crystals to the funnel, then wash the phthalic acid in the. Crystallization can be a slow process, and impatience can lead to low recovery. Use a lower temperature bath to try and encourage crystal formation. If the compound has dissolved in boiling solvent (either 0.25 or 0.5 ml), cool the. Why Is An Ice Bath Used For Crystallization.
From www.pinterest.com
Water Safe Crystals for Crystal Water and Crystal Baths — Freeing the Why Is An Ice Bath Used For Crystallization The difference in crystal lattice energy between pure and impure solids is marginal, so a solution must be cooled slowly to allow for differentiation. Place a disk of filter paper in the funnel and collect the crystals by vacuum filtration. Recrystallisation may be done in a multitude of ways, depending on your particular situation; You may use a spatula to. Why Is An Ice Bath Used For Crystallization.
From www.myglobalviewpoint.com
Ice Bath Recovery Everything You Need to Know Why Is An Ice Bath Used For Crystallization If the compound has dissolved in boiling solvent (either 0.25 or 0.5 ml), cool the tube in cold water to see if crystallization occurs. Use a lower temperature bath to try and encourage crystal formation. If a hot solution is plunged immediately into an. Recrystallisation may be done in a multitude of ways, depending on your particular situation; You may. Why Is An Ice Bath Used For Crystallization.
From made4fighters.com
Master the Art of Ice Bath Recovery A Comprehensive Guide by Made4Fighters Why Is An Ice Bath Used For Crystallization The product may be washed with ice. Recrystallisation may be done in a multitude of ways, depending on your particular situation; The difference in crystal lattice energy between pure and impure solids is marginal, so a solution must be cooled slowly to allow for differentiation. Place a disk of filter paper in the funnel and collect the crystals by vacuum. Why Is An Ice Bath Used For Crystallization.
From chemistrycake.blogspot.com
Cambridge CIE/IGCSE Chemistry Contents Why Is An Ice Bath Used For Crystallization Recrystallisation may be done in a multitude of ways, depending on your particular situation; If the compound has dissolved in boiling solvent (either 0.25 or 0.5 ml), cool the tube in cold water to see if crystallization occurs. You may use a spatula to assist in transferring the crystals to the funnel, then wash the phthalic acid in the. Crystallization. Why Is An Ice Bath Used For Crystallization.
From thecoldpod.com
Understanding Mechanism and Benefits of Ice Bathing Why Is An Ice Bath Used For Crystallization Place a disk of filter paper in the funnel and collect the crystals by vacuum filtration. You may use a spatula to assist in transferring the crystals to the funnel, then wash the phthalic acid in the. If no crystals form even after the solution has been cooled in an ice bath, take a fire polished stirring rod and etch. Why Is An Ice Bath Used For Crystallization.
From www.slideserve.com
PPT Recrystallization Lab PowerPoint Presentation ID830188 Why Is An Ice Bath Used For Crystallization The difference in crystal lattice energy between pure and impure solids is marginal, so a solution must be cooled slowly to allow for differentiation. Crystallization can be a slow process, and impatience can lead to low recovery. Recrystallisation may be done in a multitude of ways, depending on your particular situation; If the compound has dissolved in boiling solvent (either. Why Is An Ice Bath Used For Crystallization.
From holisticallysane.com
Ice baths vs cryotherapy Learn the differences Why Is An Ice Bath Used For Crystallization Recrystallisation may be done in a multitude of ways, depending on your particular situation; The product may be washed with ice. If the compound has dissolved in boiling solvent (either 0.25 or 0.5 ml), cool the tube in cold water to see if crystallization occurs. Place a disk of filter paper in the funnel and collect the crystals by vacuum. Why Is An Ice Bath Used For Crystallization.
From www.triumph-physio.co.nz
What Is The Science Behind Ice Baths? Why Is An Ice Bath Used For Crystallization If no crystals form even after the solution has been cooled in an ice bath, take a fire polished stirring rod and etch (scratch) the glass of your. Recrystallisation may be done in a multitude of ways, depending on your particular situation; The difference in crystal lattice energy between pure and impure solids is marginal, so a solution must be. Why Is An Ice Bath Used For Crystallization.
From foundspace.com.au
Ice Bath Found—Space Why Is An Ice Bath Used For Crystallization Crystallization can be a slow process, and impatience can lead to low recovery. The difference in crystal lattice energy between pure and impure solids is marginal, so a solution must be cooled slowly to allow for differentiation. Place a disk of filter paper in the funnel and collect the crystals by vacuum filtration. If the compound has dissolved in boiling. Why Is An Ice Bath Used For Crystallization.
From www.aakash.ac.in
What are crystallisation? Definition, Types and Importance chemistry Why Is An Ice Bath Used For Crystallization If the compound has dissolved in boiling solvent (either 0.25 or 0.5 ml), cool the tube in cold water to see if crystallization occurs. Crystallization can be a slow process, and impatience can lead to low recovery. The difference in crystal lattice energy between pure and impure solids is marginal, so a solution must be cooled slowly to allow for. Why Is An Ice Bath Used For Crystallization.
From www.mdpi.com
Crystals Free FullText The Effect of Ultrasound on the Why Is An Ice Bath Used For Crystallization The product may be washed with ice. The difference in crystal lattice energy between pure and impure solids is marginal, so a solution must be cooled slowly to allow for differentiation. If a hot solution is plunged immediately into an. Use a lower temperature bath to try and encourage crystal formation. You may use a spatula to assist in transferring. Why Is An Ice Bath Used For Crystallization.
From scienceinfo.com
Crystallization 4 Types, Processes, Steps, Important Applications Why Is An Ice Bath Used For Crystallization If a hot solution is plunged immediately into an. Place a disk of filter paper in the funnel and collect the crystals by vacuum filtration. The product may be washed with ice. Use a lower temperature bath to try and encourage crystal formation. Recrystallisation may be done in a multitude of ways, depending on your particular situation; If no crystals. Why Is An Ice Bath Used For Crystallization.
From www.teachoo.com
What Does One Mean By Water Of Crystallisation? Give Example Teachoo Why Is An Ice Bath Used For Crystallization You may use a spatula to assist in transferring the crystals to the funnel, then wash the phthalic acid in the. Crystallization can be a slow process, and impatience can lead to low recovery. If no crystals form even after the solution has been cooled in an ice bath, take a fire polished stirring rod and etch (scratch) the glass. Why Is An Ice Bath Used For Crystallization.
From psiberg.com
Recrystallization Types, Procedure, Applications PSIBERG Why Is An Ice Bath Used For Crystallization You may use a spatula to assist in transferring the crystals to the funnel, then wash the phthalic acid in the. Crystallization can be a slow process, and impatience can lead to low recovery. Recrystallisation may be done in a multitude of ways, depending on your particular situation; Use a lower temperature bath to try and encourage crystal formation. If. Why Is An Ice Bath Used For Crystallization.
From nordicway.shop
Ice Bath Benefits The 7 Physical Benefits of Water Cold Water Why Is An Ice Bath Used For Crystallization You may use a spatula to assist in transferring the crystals to the funnel, then wash the phthalic acid in the. Use a lower temperature bath to try and encourage crystal formation. If a hot solution is plunged immediately into an. If the compound has dissolved in boiling solvent (either 0.25 or 0.5 ml), cool the tube in cold water. Why Is An Ice Bath Used For Crystallization.
From sunhomesaunas.com
Ice Baths for Chronic Inflammation EvidenceBased Approach Sun Home Why Is An Ice Bath Used For Crystallization The product may be washed with ice. The difference in crystal lattice energy between pure and impure solids is marginal, so a solution must be cooled slowly to allow for differentiation. If no crystals form even after the solution has been cooled in an ice bath, take a fire polished stirring rod and etch (scratch) the glass of your. Place. Why Is An Ice Bath Used For Crystallization.
From evidencebasedmuscle.com
Why Ice Baths for Fat Loss Aren't Effective EVIDENCE BASED MUSCLE Why Is An Ice Bath Used For Crystallization Use a lower temperature bath to try and encourage crystal formation. The difference in crystal lattice energy between pure and impure solids is marginal, so a solution must be cooled slowly to allow for differentiation. Place a disk of filter paper in the funnel and collect the crystals by vacuum filtration. Crystallization can be a slow process, and impatience can. Why Is An Ice Bath Used For Crystallization.
From informacionpublica.svet.gob.gt
Benefits Of Ice Baths For Muscles informacionpublica.svet.gob.gt Why Is An Ice Bath Used For Crystallization If a hot solution is plunged immediately into an. If no crystals form even after the solution has been cooled in an ice bath, take a fire polished stirring rod and etch (scratch) the glass of your. Recrystallisation may be done in a multitude of ways, depending on your particular situation; Place a disk of filter paper in the funnel. Why Is An Ice Bath Used For Crystallization.
From julietsobel.blogspot.com
Juliet's Chemistry Blog Constructing A Solubility Curve Lab Why Is An Ice Bath Used For Crystallization Crystallization can be a slow process, and impatience can lead to low recovery. If the compound has dissolved in boiling solvent (either 0.25 or 0.5 ml), cool the tube in cold water to see if crystallization occurs. Recrystallisation may be done in a multitude of ways, depending on your particular situation; Place a disk of filter paper in the funnel. Why Is An Ice Bath Used For Crystallization.
From www.youtube.com
ICE BATH THERAPY THE BENEFITS OF COLD EXPOSURE (9.28.18) YouTube Why Is An Ice Bath Used For Crystallization The difference in crystal lattice energy between pure and impure solids is marginal, so a solution must be cooled slowly to allow for differentiation. If a hot solution is plunged immediately into an. Recrystallisation may be done in a multitude of ways, depending on your particular situation; If no crystals form even after the solution has been cooled in an. Why Is An Ice Bath Used For Crystallization.
From collegedunia.com
Crystallization Definition, Process, Types & Examples Why Is An Ice Bath Used For Crystallization Recrystallisation may be done in a multitude of ways, depending on your particular situation; You may use a spatula to assist in transferring the crystals to the funnel, then wash the phthalic acid in the. If no crystals form even after the solution has been cooled in an ice bath, take a fire polished stirring rod and etch (scratch) the. Why Is An Ice Bath Used For Crystallization.
From lulelaboratory.blogspot.com
Lu Le Laboratory Crystallization Techniques in Organic Chemistry Why Is An Ice Bath Used For Crystallization You may use a spatula to assist in transferring the crystals to the funnel, then wash the phthalic acid in the. Place a disk of filter paper in the funnel and collect the crystals by vacuum filtration. If the compound has dissolved in boiling solvent (either 0.25 or 0.5 ml), cool the tube in cold water to see if crystallization. Why Is An Ice Bath Used For Crystallization.
From www.getdistributors.com
8 Reasons Why Ice Bath Therapy Works Wonders GetDistributors Why Is An Ice Bath Used For Crystallization You may use a spatula to assist in transferring the crystals to the funnel, then wash the phthalic acid in the. If no crystals form even after the solution has been cooled in an ice bath, take a fire polished stirring rod and etch (scratch) the glass of your. The product may be washed with ice. If the compound has. Why Is An Ice Bath Used For Crystallization.
From www.bergenchiro.com
The Cold Truth Why Ice Baths Are The Next Big Thing in Wellness Why Is An Ice Bath Used For Crystallization Crystallization can be a slow process, and impatience can lead to low recovery. You may use a spatula to assist in transferring the crystals to the funnel, then wash the phthalic acid in the. The product may be washed with ice. Use a lower temperature bath to try and encourage crystal formation. If no crystals form even after the solution. Why Is An Ice Bath Used For Crystallization.
From www.numerade.com
SOLVED During the crystallization of acetaminophen, why was the Why Is An Ice Bath Used For Crystallization The product may be washed with ice. Recrystallisation may be done in a multitude of ways, depending on your particular situation; Use a lower temperature bath to try and encourage crystal formation. If a hot solution is plunged immediately into an. You may use a spatula to assist in transferring the crystals to the funnel, then wash the phthalic acid. Why Is An Ice Bath Used For Crystallization.
From www.slideshare.net
Purification Of Substances 1 Why Is An Ice Bath Used For Crystallization The product may be washed with ice. If a hot solution is plunged immediately into an. You may use a spatula to assist in transferring the crystals to the funnel, then wash the phthalic acid in the. The difference in crystal lattice energy between pure and impure solids is marginal, so a solution must be cooled slowly to allow for. Why Is An Ice Bath Used For Crystallization.
From ar.inspiredpencil.com
Ice Bath Chemistry Why Is An Ice Bath Used For Crystallization If the compound has dissolved in boiling solvent (either 0.25 or 0.5 ml), cool the tube in cold water to see if crystallization occurs. If no crystals form even after the solution has been cooled in an ice bath, take a fire polished stirring rod and etch (scratch) the glass of your. The product may be washed with ice. If. Why Is An Ice Bath Used For Crystallization.
From byjus.com
Crystallization Definition, Process, Separation Technique, FAQs Why Is An Ice Bath Used For Crystallization Recrystallisation may be done in a multitude of ways, depending on your particular situation; You may use a spatula to assist in transferring the crystals to the funnel, then wash the phthalic acid in the. If the compound has dissolved in boiling solvent (either 0.25 or 0.5 ml), cool the tube in cold water to see if crystallization occurs. The. Why Is An Ice Bath Used For Crystallization.
From hxemljtcp.blob.core.windows.net
Why Are Ice Baths Good For Runners at Sherry Sell blog Why Is An Ice Bath Used For Crystallization You may use a spatula to assist in transferring the crystals to the funnel, then wash the phthalic acid in the. Place a disk of filter paper in the funnel and collect the crystals by vacuum filtration. The product may be washed with ice. The difference in crystal lattice energy between pure and impure solids is marginal, so a solution. Why Is An Ice Bath Used For Crystallization.
From www.animalia-life.club
Crystallization Experiment Why Is An Ice Bath Used For Crystallization The difference in crystal lattice energy between pure and impure solids is marginal, so a solution must be cooled slowly to allow for differentiation. If the compound has dissolved in boiling solvent (either 0.25 or 0.5 ml), cool the tube in cold water to see if crystallization occurs. You may use a spatula to assist in transferring the crystals to. Why Is An Ice Bath Used For Crystallization.