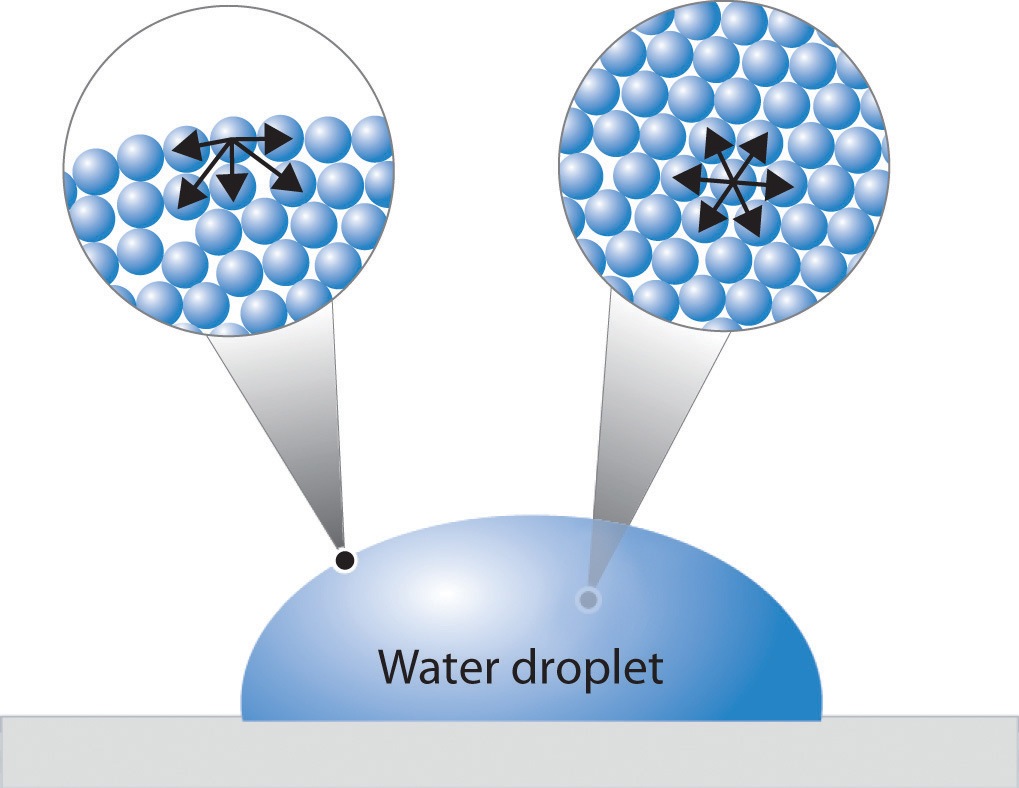
Surface Tension Facts Physics . According to the definition of surface tension, it is the phenomenon that occurs when the surface of a liquid is in contact with another phase (it can be a liquid as well). Surface tension is measured in force per unit length (n/m). Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. It is caused by cohesive forces between liquid molecules. Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. For example, the molecules of a water droplet are held together by cohesive forces, and the especially strong cohesive forces at the surface constitute surface tension. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. The molecules on the surface of a liquid are attracted by their neighbors from the.
from chem.libretexts.org
For example, the molecules of a water droplet are held together by cohesive forces, and the especially strong cohesive forces at the surface constitute surface tension. According to the definition of surface tension, it is the phenomenon that occurs when the surface of a liquid is in contact with another phase (it can be a liquid as well). Surface tension is measured in force per unit length (n/m). It is caused by cohesive forces between liquid molecules. The molecules on the surface of a liquid are attracted by their neighbors from the. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules.
Surface Tension Chemistry LibreTexts
Surface Tension Facts Physics For example, the molecules of a water droplet are held together by cohesive forces, and the especially strong cohesive forces at the surface constitute surface tension. For example, the molecules of a water droplet are held together by cohesive forces, and the especially strong cohesive forces at the surface constitute surface tension. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. The molecules on the surface of a liquid are attracted by their neighbors from the. Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. Surface tension is measured in force per unit length (n/m). It is caused by cohesive forces between liquid molecules. According to the definition of surface tension, it is the phenomenon that occurs when the surface of a liquid is in contact with another phase (it can be a liquid as well). Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane.
From www.youtube.com
Surface Tension on the basis of Molecular theory YouTube Surface Tension Facts Physics Surface tension is measured in force per unit length (n/m). According to the definition of surface tension, it is the phenomenon that occurs when the surface of a liquid is in contact with another phase (it can be a liquid as well). For example, the molecules of a water droplet are held together by cohesive forces, and the especially strong. Surface Tension Facts Physics.
From chem.libretexts.org
Surface Tension Chemistry LibreTexts Surface Tension Facts Physics “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. The molecules on the surface of a liquid are attracted by their neighbors from the. It is caused by cohesive forces between liquid molecules. Surface tension is a phenomenon. Surface Tension Facts Physics.
From byjus.com
Explain the surface tension phenomenon with examples. Surface Tension Facts Physics For example, the molecules of a water droplet are held together by cohesive forces, and the especially strong cohesive forces at the surface constitute surface tension. According to the definition of surface tension, it is the phenomenon that occurs when the surface of a liquid is in contact with another phase (it can be a liquid as well). Surface tension,. Surface Tension Facts Physics.
From www.youtube.com
The Meaning of Surface Tension and its Practical Applications YouTube Surface Tension Facts Physics For example, the molecules of a water droplet are held together by cohesive forces, and the especially strong cohesive forces at the surface constitute surface tension. Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. According to the definition of surface tension, it is the phenomenon that occurs when the surface of a liquid. Surface Tension Facts Physics.
From www.science-sparks.com
Surface Tension of Water Science Experiments for Kids Surface Tension Facts Physics It is caused by cohesive forces between liquid molecules. Surface tension is measured in force per unit length (n/m). According to the definition of surface tension, it is the phenomenon that occurs when the surface of a liquid is in contact with another phase (it can be a liquid as well). The molecules on the surface of a liquid are. Surface Tension Facts Physics.
From www.slideshare.net
Understanding Surface Tension Part 1 12th Physics Surface Tension Facts Physics According to the definition of surface tension, it is the phenomenon that occurs when the surface of a liquid is in contact with another phase (it can be a liquid as well). It is caused by cohesive forces between liquid molecules. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of. Surface Tension Facts Physics.
From study.com
Surface Tension Definition, Calculation & Examples Video & Lesson Surface Tension Facts Physics Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. It is caused by cohesive forces between liquid molecules. Surface tension is measured in force per unit length (n/m). The molecules on the surface of a liquid are attracted by their neighbors from the. Surface tension, property of a liquid surface displayed by its acting. Surface Tension Facts Physics.
From www.britannica.com
surface tension Definition, Examples, & Facts Surface Tension Facts Physics “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. The molecules on the surface of a liquid are attracted by their neighbors from the. Surface tension, property of a liquid surface displayed by its acting as if it. Surface Tension Facts Physics.
From scienceinfo.com
Surface Tension Definition, Formula, Unit, Causes, Examples, Consequences Surface Tension Facts Physics Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. The molecules on the surface of a liquid are attracted by their neighbors from the. For example, the molecules of a water droplet are held together by cohesive forces, and the especially strong cohesive forces at the surface constitute surface tension. Surface tension, property of. Surface Tension Facts Physics.
From stock.adobe.com
illustration of physics, Surface tension of water, the cohesive forces Surface Tension Facts Physics “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. According to the definition of surface tension, it is the phenomenon that occurs when the surface of a liquid is in contact with another phase (it can be a. Surface Tension Facts Physics.
From studiousguy.com
10 Surface Tension Examples in Daily Life StudiousGuy Surface Tension Facts Physics Surface tension is measured in force per unit length (n/m). The molecules on the surface of a liquid are attracted by their neighbors from the. Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. According to the definition of surface tension, it is the phenomenon that occurs when the surface of a liquid is. Surface Tension Facts Physics.
From techblog.ctgclean.com
What is Surface Tension? CTG Technical Blog Surface Tension Facts Physics “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. The molecules on the surface of a liquid are attracted by their neighbors from the. According to the definition of surface tension, it is the phenomenon that occurs when. Surface Tension Facts Physics.
From www.youtube.com
Class 11 Physics Surface tension 4 Important facts about surface Surface Tension Facts Physics It is caused by cohesive forces between liquid molecules. For example, the molecules of a water droplet are held together by cohesive forces, and the especially strong cohesive forces at the surface constitute surface tension. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer,. Surface Tension Facts Physics.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance Surface Tension Facts Physics Surface tension is measured in force per unit length (n/m). Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. According to the definition. Surface Tension Facts Physics.
From schematicpartebriose.z14.web.core.windows.net
Surface Tension Simple Explanation Surface Tension Facts Physics “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. The molecules on the surface of a liquid are attracted by their neighbors from the. For example, the molecules of a water droplet are held together by cohesive forces,. Surface Tension Facts Physics.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs Surface Tension Facts Physics “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. For example, the molecules of a water droplet are held together by cohesive forces, and the especially strong cohesive forces at the surface constitute surface tension. Surface tension is. Surface Tension Facts Physics.
From learnminepic.blogspot.com
The Best 30 Surface Tension Definition Physics learnminepic Surface Tension Facts Physics Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. It is caused by cohesive forces between liquid molecules. Surface tension is measured in force per unit length (n/m). For example, the molecules of a water droplet are held together by cohesive forces, and the especially strong cohesive forces at the surface constitute surface tension.. Surface Tension Facts Physics.
From byjus.com
what is surface tension and surface energy? Surface Tension Facts Physics The molecules on the surface of a liquid are attracted by their neighbors from the. According to the definition of surface tension, it is the phenomenon that occurs when the surface of a liquid is in contact with another phase (it can be a liquid as well). Surface tension is measured in force per unit length (n/m). “surface tension is. Surface Tension Facts Physics.
From www.slideserve.com
PPT Surface Tension PowerPoint Presentation, free download ID6898122 Surface Tension Facts Physics The molecules on the surface of a liquid are attracted by their neighbors from the. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Surface tension is measured in force per unit length (n/m). For example, the molecules of a water droplet are held together by cohesive forces, and the. Surface Tension Facts Physics.
From upberi.com
Surface Tension Definition, Formula, Causes, Examples, and FAQs (2023) Surface Tension Facts Physics Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. It is caused by cohesive forces between liquid molecules. For example, the molecules of a water droplet are held together by cohesive forces, and the especially. Surface Tension Facts Physics.
From www.worksheetsplanet.com
What is Surface Tension? Surface Tension Facts Physics It is caused by cohesive forces between liquid molecules. The molecules on the surface of a liquid are attracted by their neighbors from the. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. Surface tension, property of a. Surface Tension Facts Physics.
From www.dreamstime.com
Surface Tension Explanation Vector Illustration Diagram Stock Vector Surface Tension Facts Physics According to the definition of surface tension, it is the phenomenon that occurs when the surface of a liquid is in contact with another phase (it can be a liquid as well). Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Surface tension is a phenomenon that occurs due to. Surface Tension Facts Physics.
From www.sciencefacts.net
Surface Tension Definition, Examples, and Unit Surface Tension Facts Physics Surface tension is measured in force per unit length (n/m). According to the definition of surface tension, it is the phenomenon that occurs when the surface of a liquid is in contact with another phase (it can be a liquid as well). “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction. Surface Tension Facts Physics.
From www.youtube.com
Chapter7.6 Surface Tension Complete 11th Class Physics Notes YouTube Surface Tension Facts Physics Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. For example, the molecules of a water droplet are held together by cohesive forces, and the especially strong cohesive forces at the surface constitute surface tension. Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules.. Surface Tension Facts Physics.
From chemistnotes.com
Surface Tension Definition, Units, Epic Examples, Effects, and Surface Tension Facts Physics Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. For example, the molecules of a water droplet are held together by cohesive forces, and the especially strong cohesive forces at the surface constitute surface tension. The molecules on the surface of a liquid are attracted by their neighbors from the.. Surface Tension Facts Physics.
From www.slideserve.com
PPT Chapter 15 PowerPoint Presentation ID245553 Surface Tension Facts Physics According to the definition of surface tension, it is the phenomenon that occurs when the surface of a liquid is in contact with another phase (it can be a liquid as well). It is caused by cohesive forces between liquid molecules. The molecules on the surface of a liquid are attracted by their neighbors from the. “surface tension is the. Surface Tension Facts Physics.
From www.aakash.ac.in
Surface Tension Definition, Causes, Measurement & Formula AESL Surface Tension Facts Physics For example, the molecules of a water droplet are held together by cohesive forces, and the especially strong cohesive forces at the surface constitute surface tension. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. The molecules on. Surface Tension Facts Physics.
From www.youtube.com
PHYSICS DERIVATION OF SURFACE TENSION YouTube Surface Tension Facts Physics The molecules on the surface of a liquid are attracted by their neighbors from the. For example, the molecules of a water droplet are held together by cohesive forces, and the especially strong cohesive forces at the surface constitute surface tension. According to the definition of surface tension, it is the phenomenon that occurs when the surface of a liquid. Surface Tension Facts Physics.
From www.aakash.ac.in
Surface Tension Definition, Causes, Measurement & Formula AESL Surface Tension Facts Physics According to the definition of surface tension, it is the phenomenon that occurs when the surface of a liquid is in contact with another phase (it can be a liquid as well). Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. The molecules on the surface of a liquid are attracted by their neighbors. Surface Tension Facts Physics.
From eduinput.com
Surface TensionDefinition, Examples, Causes, And Measurement Surface Tension Facts Physics The molecules on the surface of a liquid are attracted by their neighbors from the. For example, the molecules of a water droplet are held together by cohesive forces, and the especially strong cohesive forces at the surface constitute surface tension. Surface tension is measured in force per unit length (n/m). According to the definition of surface tension, it is. Surface Tension Facts Physics.
From www.youtube.com
Surface Tension What is it, how does it form, what properties does it Surface Tension Facts Physics The molecules on the surface of a liquid are attracted by their neighbors from the. Surface tension is measured in force per unit length (n/m). Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. “surface tension is the tension of a liquid’s surface film caused by the bulk of the. Surface Tension Facts Physics.
From www.bigstockphoto.com
Surface Tension Image & Photo (Free Trial) Bigstock Surface Tension Facts Physics Surface tension is measured in force per unit length (n/m). According to the definition of surface tension, it is the phenomenon that occurs when the surface of a liquid is in contact with another phase (it can be a liquid as well). Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. For example, the. Surface Tension Facts Physics.
From www.learnatnoon.com
What is Surface Tension in Physics? Surface Tension Facts Physics “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. The molecules on the surface of a liquid are attracted by their neighbors from the. Surface tension, property of a liquid surface displayed by its acting as if it. Surface Tension Facts Physics.
From www.slideserve.com
PPT Intermolecular Forces and Liquids and Solids PowerPoint Surface Tension Facts Physics The molecules on the surface of a liquid are attracted by their neighbors from the. Surface tension is measured in force per unit length (n/m). It is caused by cohesive forces between liquid molecules. According to the definition of surface tension, it is the phenomenon that occurs when the surface of a liquid is in contact with another phase (it. Surface Tension Facts Physics.
From www.slideserve.com
PPT Surface Tension PowerPoint Presentation, free download ID3106425 Surface Tension Facts Physics “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. Surface tension is measured in force per unit length (n/m). The molecules on the surface of a liquid are attracted by their neighbors from the. Surface tension, property of. Surface Tension Facts Physics.