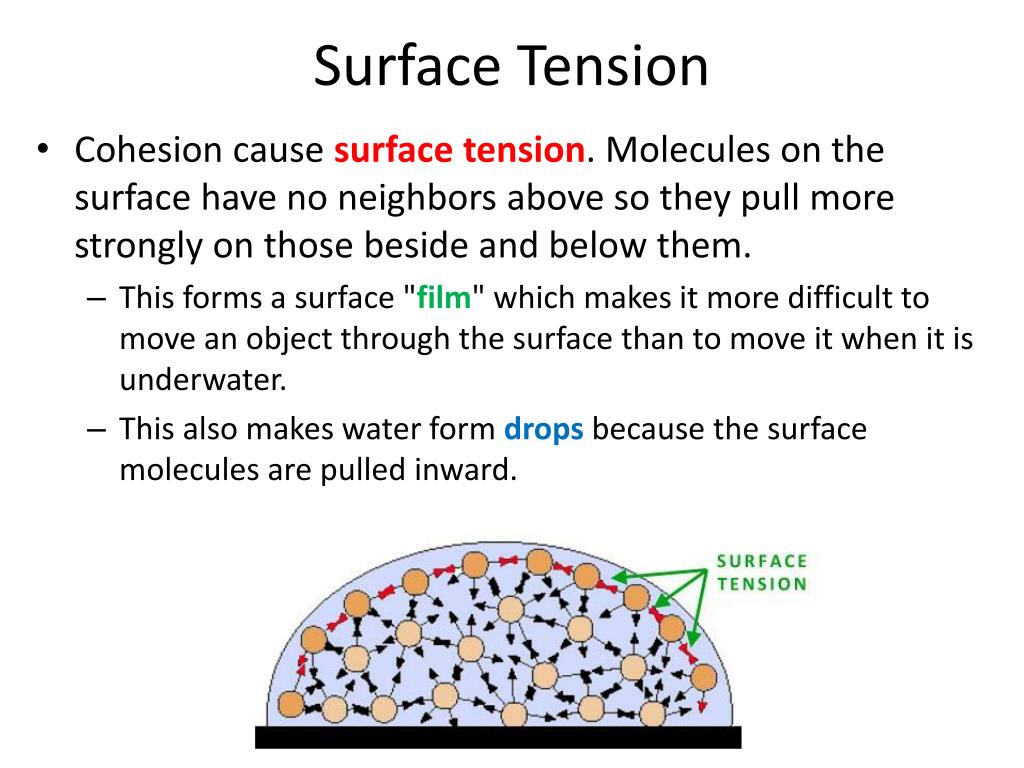
Water Surface Tension Polar Molecule . Students will relate these observations to an explanation of surface tension at the molecular level. Read this tutorial to know why water is polar! Surface tension is the force that allows a liquid to resist external forces, and it's high in polar substances like water due to the strong. Cohesion is intermolecular forces between like molecules; Evidence of water's surface tension can be seen where the water strider's legs dent but do not break through the water's surface. The attraction of polar water molecules to each other. Water consists of one oxygen atom flanked by two hydrogen atoms. This is why water molecules are able to hold themselves together in a drop. Water has high surface tension, which can be explained by its polarity and hydrogen bonding. We will provide you with the basics of polarity, as well as what polarity means for water (e.g. An example of such an organism is the water strider, which can run across the surface of water, due to the intermolecular forces of the molecules, and the force of the strider which is distributed to.
from mavink.com
This is why water molecules are able to hold themselves together in a drop. An example of such an organism is the water strider, which can run across the surface of water, due to the intermolecular forces of the molecules, and the force of the strider which is distributed to. The attraction of polar water molecules to each other. Water consists of one oxygen atom flanked by two hydrogen atoms. Water has high surface tension, which can be explained by its polarity and hydrogen bonding. Evidence of water's surface tension can be seen where the water strider's legs dent but do not break through the water's surface. Cohesion is intermolecular forces between like molecules; Students will relate these observations to an explanation of surface tension at the molecular level. Read this tutorial to know why water is polar! We will provide you with the basics of polarity, as well as what polarity means for water (e.g.
Water Molecules Surface Tension
Water Surface Tension Polar Molecule We will provide you with the basics of polarity, as well as what polarity means for water (e.g. Evidence of water's surface tension can be seen where the water strider's legs dent but do not break through the water's surface. Students will relate these observations to an explanation of surface tension at the molecular level. Surface tension is the force that allows a liquid to resist external forces, and it's high in polar substances like water due to the strong. An example of such an organism is the water strider, which can run across the surface of water, due to the intermolecular forces of the molecules, and the force of the strider which is distributed to. This is why water molecules are able to hold themselves together in a drop. Water consists of one oxygen atom flanked by two hydrogen atoms. Water has high surface tension, which can be explained by its polarity and hydrogen bonding. The attraction of polar water molecules to each other. Cohesion is intermolecular forces between like molecules; Read this tutorial to know why water is polar! We will provide you with the basics of polarity, as well as what polarity means for water (e.g.
From nl.pinterest.com
Why Is Water a Polar Molecule? Hydrogen Atom, Hydrogen Bond, Ionic Water Surface Tension Polar Molecule We will provide you with the basics of polarity, as well as what polarity means for water (e.g. Surface tension is the force that allows a liquid to resist external forces, and it's high in polar substances like water due to the strong. The attraction of polar water molecules to each other. This is why water molecules are able to. Water Surface Tension Polar Molecule.
From mavink.com
Polar Water Molecule Diagram Water Surface Tension Polar Molecule Students will relate these observations to an explanation of surface tension at the molecular level. Read this tutorial to know why water is polar! We will provide you with the basics of polarity, as well as what polarity means for water (e.g. Water has high surface tension, which can be explained by its polarity and hydrogen bonding. Cohesion is intermolecular. Water Surface Tension Polar Molecule.
From mungfali.com
Polar Water Molecule Diagram Water Surface Tension Polar Molecule Evidence of water's surface tension can be seen where the water strider's legs dent but do not break through the water's surface. An example of such an organism is the water strider, which can run across the surface of water, due to the intermolecular forces of the molecules, and the force of the strider which is distributed to. Water consists. Water Surface Tension Polar Molecule.
From hebasoffar.blogspot.com
Science online The importance of the water and its structure Water Surface Tension Polar Molecule The attraction of polar water molecules to each other. An example of such an organism is the water strider, which can run across the surface of water, due to the intermolecular forces of the molecules, and the force of the strider which is distributed to. Water consists of one oxygen atom flanked by two hydrogen atoms. Evidence of water's surface. Water Surface Tension Polar Molecule.
From vdocuments.mx
Properties of Water. Polar molecule Cohesion and adhesion High specific Water Surface Tension Polar Molecule Read this tutorial to know why water is polar! Water consists of one oxygen atom flanked by two hydrogen atoms. This is why water molecules are able to hold themselves together in a drop. The attraction of polar water molecules to each other. An example of such an organism is the water strider, which can run across the surface of. Water Surface Tension Polar Molecule.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance Water Surface Tension Polar Molecule The attraction of polar water molecules to each other. An example of such an organism is the water strider, which can run across the surface of water, due to the intermolecular forces of the molecules, and the force of the strider which is distributed to. Water consists of one oxygen atom flanked by two hydrogen atoms. We will provide you. Water Surface Tension Polar Molecule.
From www.slideserve.com
PPT Understanding Water PowerPoint Presentation, free download ID Water Surface Tension Polar Molecule This is why water molecules are able to hold themselves together in a drop. The attraction of polar water molecules to each other. We will provide you with the basics of polarity, as well as what polarity means for water (e.g. Water has high surface tension, which can be explained by its polarity and hydrogen bonding. Students will relate these. Water Surface Tension Polar Molecule.
From courses.lumenlearning.com
Properties of Liquids Chemistry Atoms First Water Surface Tension Polar Molecule An example of such an organism is the water strider, which can run across the surface of water, due to the intermolecular forces of the molecules, and the force of the strider which is distributed to. This is why water molecules are able to hold themselves together in a drop. We will provide you with the basics of polarity, as. Water Surface Tension Polar Molecule.
From stock.adobe.com
illustration of physics, Surface tension of water, the cohesive forces Water Surface Tension Polar Molecule Read this tutorial to know why water is polar! Students will relate these observations to an explanation of surface tension at the molecular level. This is why water molecules are able to hold themselves together in a drop. We will provide you with the basics of polarity, as well as what polarity means for water (e.g. Evidence of water's surface. Water Surface Tension Polar Molecule.
From www.acs.org
Simulations & Videos for Lesson 5.2 Surface Tension American Water Surface Tension Polar Molecule An example of such an organism is the water strider, which can run across the surface of water, due to the intermolecular forces of the molecules, and the force of the strider which is distributed to. This is why water molecules are able to hold themselves together in a drop. Read this tutorial to know why water is polar! Evidence. Water Surface Tension Polar Molecule.
From www.dreamstime.com
The Polar Covalent Bonds of Water Molecules (H2O) Stock Vector Water Surface Tension Polar Molecule This is why water molecules are able to hold themselves together in a drop. Cohesion is intermolecular forces between like molecules; Surface tension is the force that allows a liquid to resist external forces, and it's high in polar substances like water due to the strong. We will provide you with the basics of polarity, as well as what polarity. Water Surface Tension Polar Molecule.
From www.usgs.gov
The strong polar bond between water molecules creates water cohesion. Water Surface Tension Polar Molecule Cohesion is intermolecular forces between like molecules; This is why water molecules are able to hold themselves together in a drop. Water consists of one oxygen atom flanked by two hydrogen atoms. An example of such an organism is the water strider, which can run across the surface of water, due to the intermolecular forces of the molecules, and the. Water Surface Tension Polar Molecule.
From www.slideserve.com
PPT Properties of Water PowerPoint Presentation, free download ID Water Surface Tension Polar Molecule Water consists of one oxygen atom flanked by two hydrogen atoms. We will provide you with the basics of polarity, as well as what polarity means for water (e.g. Cohesion is intermolecular forces between like molecules; Water has high surface tension, which can be explained by its polarity and hydrogen bonding. Read this tutorial to know why water is polar!. Water Surface Tension Polar Molecule.
From www.biolinscientific.com
Surface tension of water Why is it so high? Water Surface Tension Polar Molecule Read this tutorial to know why water is polar! Cohesion is intermolecular forces between like molecules; We will provide you with the basics of polarity, as well as what polarity means for water (e.g. The attraction of polar water molecules to each other. This is why water molecules are able to hold themselves together in a drop. Evidence of water's. Water Surface Tension Polar Molecule.
From mavink.com
Water Molecules Surface Tension Water Surface Tension Polar Molecule Water has high surface tension, which can be explained by its polarity and hydrogen bonding. Cohesion is intermolecular forces between like molecules; This is why water molecules are able to hold themselves together in a drop. We will provide you with the basics of polarity, as well as what polarity means for water (e.g. Water consists of one oxygen atom. Water Surface Tension Polar Molecule.
From www.thoughtco.com
Why Is Water a Polar Molecule? Water Surface Tension Polar Molecule Read this tutorial to know why water is polar! Evidence of water's surface tension can be seen where the water strider's legs dent but do not break through the water's surface. Water has high surface tension, which can be explained by its polarity and hydrogen bonding. Water consists of one oxygen atom flanked by two hydrogen atoms. Surface tension is. Water Surface Tension Polar Molecule.
From sophisticatedthoughtscom.wordpress.com
The Science Behind Bubbles (1/3) Sophisticated Thoughts Water Surface Tension Polar Molecule Read this tutorial to know why water is polar! An example of such an organism is the water strider, which can run across the surface of water, due to the intermolecular forces of the molecules, and the force of the strider which is distributed to. Water consists of one oxygen atom flanked by two hydrogen atoms. The attraction of polar. Water Surface Tension Polar Molecule.
From www.alamy.com
Water above rim of cup surface tension caused by cohesion between the Water Surface Tension Polar Molecule Evidence of water's surface tension can be seen where the water strider's legs dent but do not break through the water's surface. Surface tension is the force that allows a liquid to resist external forces, and it's high in polar substances like water due to the strong. This is why water molecules are able to hold themselves together in a. Water Surface Tension Polar Molecule.
From www.slideserve.com
PPT Water and It’s properties PowerPoint Presentation, free download Water Surface Tension Polar Molecule The attraction of polar water molecules to each other. Evidence of water's surface tension can be seen where the water strider's legs dent but do not break through the water's surface. Surface tension is the force that allows a liquid to resist external forces, and it's high in polar substances like water due to the strong. Water has high surface. Water Surface Tension Polar Molecule.
From biologydictionary.net
Polar Molecule Definition and Examples Biology Dictionary Water Surface Tension Polar Molecule We will provide you with the basics of polarity, as well as what polarity means for water (e.g. This is why water molecules are able to hold themselves together in a drop. Read this tutorial to know why water is polar! Students will relate these observations to an explanation of surface tension at the molecular level. Water has high surface. Water Surface Tension Polar Molecule.
From www.slideserve.com
PPT Marine Biology Lesson 3 PowerPoint Presentation, free download Water Surface Tension Polar Molecule Evidence of water's surface tension can be seen where the water strider's legs dent but do not break through the water's surface. Students will relate these observations to an explanation of surface tension at the molecular level. The attraction of polar water molecules to each other. Read this tutorial to know why water is polar! An example of such an. Water Surface Tension Polar Molecule.
From pdfslide.net
(PPT) Structure & Properties of Water. STRUCTURE OF WATER POLAR Water Surface Tension Polar Molecule This is why water molecules are able to hold themselves together in a drop. Students will relate these observations to an explanation of surface tension at the molecular level. Surface tension is the force that allows a liquid to resist external forces, and it's high in polar substances like water due to the strong. The attraction of polar water molecules. Water Surface Tension Polar Molecule.
From 88guru.com
Viscosity And Surface Tension Definition, Applications and FAQ's 88Guru Water Surface Tension Polar Molecule Evidence of water's surface tension can be seen where the water strider's legs dent but do not break through the water's surface. Students will relate these observations to an explanation of surface tension at the molecular level. An example of such an organism is the water strider, which can run across the surface of water, due to the intermolecular forces. Water Surface Tension Polar Molecule.
From socratic.org
What kinds of molecules are polar? + Example Water Surface Tension Polar Molecule Students will relate these observations to an explanation of surface tension at the molecular level. The attraction of polar water molecules to each other. An example of such an organism is the water strider, which can run across the surface of water, due to the intermolecular forces of the molecules, and the force of the strider which is distributed to.. Water Surface Tension Polar Molecule.
From errantscience.com
ErrantScience Water surface tension Water Surface Tension Polar Molecule Read this tutorial to know why water is polar! We will provide you with the basics of polarity, as well as what polarity means for water (e.g. Water has high surface tension, which can be explained by its polarity and hydrogen bonding. This is why water molecules are able to hold themselves together in a drop. Evidence of water's surface. Water Surface Tension Polar Molecule.
From ar.inspiredpencil.com
Surface Tension Of Water Molecules Water Surface Tension Polar Molecule Cohesion is intermolecular forces between like molecules; Water has high surface tension, which can be explained by its polarity and hydrogen bonding. Water consists of one oxygen atom flanked by two hydrogen atoms. We will provide you with the basics of polarity, as well as what polarity means for water (e.g. This is why water molecules are able to hold. Water Surface Tension Polar Molecule.
From www.vrogue.co
What Is Surface Tension Molecular Explanation vrogue.co Water Surface Tension Polar Molecule Water consists of one oxygen atom flanked by two hydrogen atoms. Water has high surface tension, which can be explained by its polarity and hydrogen bonding. The attraction of polar water molecules to each other. An example of such an organism is the water strider, which can run across the surface of water, due to the intermolecular forces of the. Water Surface Tension Polar Molecule.
From chem.libretexts.org
Surface Tension Chemistry LibreTexts Water Surface Tension Polar Molecule Read this tutorial to know why water is polar! We will provide you with the basics of polarity, as well as what polarity means for water (e.g. Water consists of one oxygen atom flanked by two hydrogen atoms. Surface tension is the force that allows a liquid to resist external forces, and it's high in polar substances like water due. Water Surface Tension Polar Molecule.
From www.slideserve.com
PPT Chapter 11 Intermolecular Forces, Liquids and Solids PowerPoint Water Surface Tension Polar Molecule Water has high surface tension, which can be explained by its polarity and hydrogen bonding. Read this tutorial to know why water is polar! Cohesion is intermolecular forces between like molecules; Water consists of one oxygen atom flanked by two hydrogen atoms. Students will relate these observations to an explanation of surface tension at the molecular level. This is why. Water Surface Tension Polar Molecule.
From www.thoughtco.com
Polar Molecule Definition and Examples Water Surface Tension Polar Molecule An example of such an organism is the water strider, which can run across the surface of water, due to the intermolecular forces of the molecules, and the force of the strider which is distributed to. Students will relate these observations to an explanation of surface tension at the molecular level. Water consists of one oxygen atom flanked by two. Water Surface Tension Polar Molecule.
From slidetodoc.com
Structure Properties of Water STRUCTURE OF WATER UNIVERSAL Water Surface Tension Polar Molecule An example of such an organism is the water strider, which can run across the surface of water, due to the intermolecular forces of the molecules, and the force of the strider which is distributed to. Cohesion is intermolecular forces between like molecules; Read this tutorial to know why water is polar! Water has high surface tension, which can be. Water Surface Tension Polar Molecule.
From www.youtube.com
Surface Tension of Water Explained YouTube Water Surface Tension Polar Molecule Water consists of one oxygen atom flanked by two hydrogen atoms. This is why water molecules are able to hold themselves together in a drop. Evidence of water's surface tension can be seen where the water strider's legs dent but do not break through the water's surface. Read this tutorial to know why water is polar! An example of such. Water Surface Tension Polar Molecule.
From philschatz.com
Chemical Bonds · Anatomy and Physiology Water Surface Tension Polar Molecule The attraction of polar water molecules to each other. Water consists of one oxygen atom flanked by two hydrogen atoms. We will provide you with the basics of polarity, as well as what polarity means for water (e.g. Read this tutorial to know why water is polar! This is why water molecules are able to hold themselves together in a. Water Surface Tension Polar Molecule.
From www.slideserve.com
PPT Chapter 15 PowerPoint Presentation, free download ID245553 Water Surface Tension Polar Molecule Cohesion is intermolecular forces between like molecules; Evidence of water's surface tension can be seen where the water strider's legs dent but do not break through the water's surface. Water consists of one oxygen atom flanked by two hydrogen atoms. This is why water molecules are able to hold themselves together in a drop. Water has high surface tension, which. Water Surface Tension Polar Molecule.
From www.acs.org
Lesson 5.1 Water is a Polar Molecule American Chemical Society Water Surface Tension Polar Molecule An example of such an organism is the water strider, which can run across the surface of water, due to the intermolecular forces of the molecules, and the force of the strider which is distributed to. The attraction of polar water molecules to each other. Read this tutorial to know why water is polar! Cohesion is intermolecular forces between like. Water Surface Tension Polar Molecule.