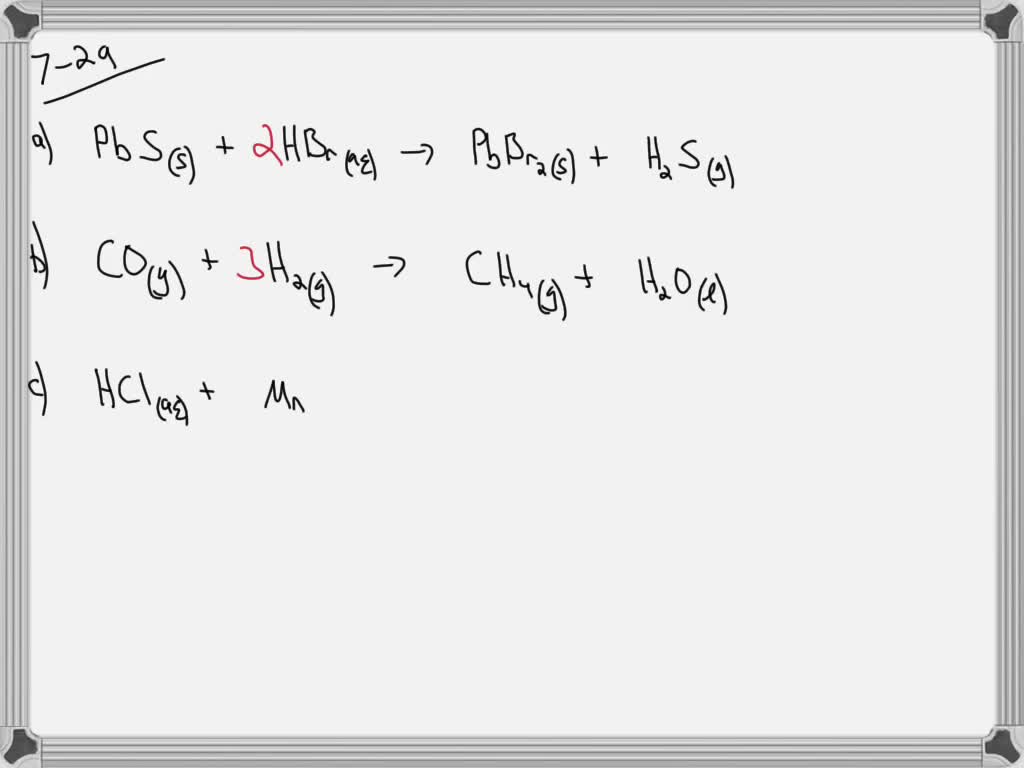Lead Ii Chloride Magnesium Balanced Equation . \[\ce{pb(no3)2(aq) + nacl(aq) → nano3(aq) + pbcl2(s)} \nonumber \] 2. \[\ce{pb(no3)2(aq) + nacl(aq) → nano3(aq) + pbcl2(s)} \nonumber \] 2. The most complex substance is lead (ii) chloride. The balanced equation will be calculated along with the solubility states,. Enter an equation of an ionic chemical equation and press the balance button. 1 atom in reagents and 2 atoms in products. The most complex substance is lead (ii) chloride. There are twice as many chloride ions in. In the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and. In order to balance mg on both sides we:. A solution of lead(ii) nitrate is mixed. 1 mg + 1 pbcl 4 = 1 pb + 2 mgcl 2 mg is not balanced: Balancing chemical equations write a balanced equation for the reaction of molecular nitrogen (n 2) and oxygen (o 2) to form dinitrogen pentoxide. Balance any equation or reaction using this chemical equation balancer! Ionic compounds do not exist as molecules.
from www.numerade.com
The balanced equation will be calculated along with the solubility states,. 1 mg + 1 pbcl 4 = 1 pb + 2 mgcl 2 mg is not balanced: \[\ce{pb(no3)2(aq) + nacl(aq) → nano3(aq) + pbcl2(s)} \nonumber \] 2. Balance any equation or reaction using this chemical equation balancer! There are twice as many chloride ions in. 1 atom in reagents and 2 atoms in products. A solution of lead(ii) nitrate is mixed. \[\ce{pb(no3)2(aq) + nacl(aq) → nano3(aq) + pbcl2(s)} \nonumber \] 2. Ionic compounds do not exist as molecules. Enter an equation of an ionic chemical equation and press the balance button.
SOLVED Write a balanced chemical equation for each reaction. a. Solid
Lead Ii Chloride Magnesium Balanced Equation In the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and. There are twice as many chloride ions in. The most complex substance is lead (ii) chloride. Find out what type of reaction occured. Balance any equation or reaction using this chemical equation balancer! 1 mg + 1 pbcl 4 = 1 pb + 2 mgcl 2 mg is not balanced: \[\ce{pb(no3)2(aq) + nacl(aq) → nano3(aq) + pbcl2(s)} \nonumber \] 2. Balancing chemical equations write a balanced equation for the reaction of molecular nitrogen (n 2) and oxygen (o 2) to form dinitrogen pentoxide. \[\ce{pb(no3)2(aq) + nacl(aq) → nano3(aq) + pbcl2(s)} \nonumber \] 2. In order to balance mg on both sides we:. A solution of lead(ii) nitrate is mixed. 1 atom in reagents and 2 atoms in products. The most complex substance is lead (ii) chloride. Enter an equation of an ionic chemical equation and press the balance button. The balanced equation will be calculated along with the solubility states,. In the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and.
From www.thesciencehive.co.uk
Mass and Mole Calculations (AQA) — the science sauce Lead Ii Chloride Magnesium Balanced Equation The most complex substance is lead (ii) chloride. Balance any equation or reaction using this chemical equation balancer! The most complex substance is lead (ii) chloride. There are twice as many chloride ions in. Enter an equation of an ionic chemical equation and press the balance button. A solution of lead(ii) nitrate is mixed. In order to balance mg on. Lead Ii Chloride Magnesium Balanced Equation.
From www.chegg.com
Solved Solid lead(II) sulfide reacts with aqueous Lead Ii Chloride Magnesium Balanced Equation \[\ce{pb(no3)2(aq) + nacl(aq) → nano3(aq) + pbcl2(s)} \nonumber \] 2. The most complex substance is lead (ii) chloride. In order to balance mg on both sides we:. Ionic compounds do not exist as molecules. 1 atom in reagents and 2 atoms in products. The most complex substance is lead (ii) chloride. Balance any equation or reaction using this chemical equation. Lead Ii Chloride Magnesium Balanced Equation.
From www.nagwa.com
Question Video Writing the Symbol and the Ionic Equations for a Lead Ii Chloride Magnesium Balanced Equation In order to balance mg on both sides we:. The most complex substance is lead (ii) chloride. In the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and. Balance any equation or reaction using this chemical equation balancer! The most complex substance is lead (ii) chloride. \[\ce{pb(no3)2(aq) + nacl(aq) → nano3(aq) + pbcl2(s)}. Lead Ii Chloride Magnesium Balanced Equation.
From sciencenotes.org
Net Ionic Equation and Complete Ionic Equation Lead Ii Chloride Magnesium Balanced Equation In order to balance mg on both sides we:. Find out what type of reaction occured. 1 atom in reagents and 2 atoms in products. Enter an equation of an ionic chemical equation and press the balance button. Ionic compounds do not exist as molecules. In the solid state, ionic compounds are in crystal lattice containing many ions each of. Lead Ii Chloride Magnesium Balanced Equation.
From loepbliqd.blob.core.windows.net
Lead Ii Nitrate Sodium Carbonate Ionic Equation at Mitchell Laxton blog Lead Ii Chloride Magnesium Balanced Equation A solution of lead(ii) nitrate is mixed. 1 atom in reagents and 2 atoms in products. There are twice as many chloride ions in. The balanced equation will be calculated along with the solubility states,. 1 mg + 1 pbcl 4 = 1 pb + 2 mgcl 2 mg is not balanced: \[\ce{pb(no3)2(aq) + nacl(aq) → nano3(aq) + pbcl2(s)} \nonumber. Lead Ii Chloride Magnesium Balanced Equation.
From www.numerade.com
SOLVED Magnesium metal reacts with chlorine gas, Cl2, to produce Lead Ii Chloride Magnesium Balanced Equation Ionic compounds do not exist as molecules. 1 atom in reagents and 2 atoms in products. There are twice as many chloride ions in. In the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and. The balanced equation will be calculated along with the solubility states,. In order to balance mg on both. Lead Ii Chloride Magnesium Balanced Equation.
From www.chegg.com
Solved An aqueous solution of lead(II) nitrate is mixed with Lead Ii Chloride Magnesium Balanced Equation There are twice as many chloride ions in. Balance any equation or reaction using this chemical equation balancer! \[\ce{pb(no3)2(aq) + nacl(aq) → nano3(aq) + pbcl2(s)} \nonumber \] 2. In order to balance mg on both sides we:. Find out what type of reaction occured. The most complex substance is lead (ii) chloride. 1 atom in reagents and 2 atoms in. Lead Ii Chloride Magnesium Balanced Equation.
From www.youtube.com
How to Write the Formula for Magnesium chlorate YouTube Lead Ii Chloride Magnesium Balanced Equation The most complex substance is lead (ii) chloride. Enter an equation of an ionic chemical equation and press the balance button. Find out what type of reaction occured. 1 mg + 1 pbcl 4 = 1 pb + 2 mgcl 2 mg is not balanced: 1 atom in reagents and 2 atoms in products. In the solid state, ionic compounds. Lead Ii Chloride Magnesium Balanced Equation.
From www.numerade.com
SOLVED Write a balanced chemical equation for each reaction. a. Solid Lead Ii Chloride Magnesium Balanced Equation In the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and. Enter an equation of an ionic chemical equation and press the balance button. 1 mg + 1 pbcl 4 = 1 pb + 2 mgcl 2 mg is not balanced: Balancing chemical equations write a balanced equation for the reaction of molecular. Lead Ii Chloride Magnesium Balanced Equation.
From www.youtube.com
How to Write the Net Ionic Equation for Mg + FeSO4 = MgSO4 + Fe YouTube Lead Ii Chloride Magnesium Balanced Equation In the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and. Ionic compounds do not exist as molecules. The balanced equation will be calculated along with the solubility states,. Balance any equation or reaction using this chemical equation balancer! Enter an equation of an ionic chemical equation and press the balance button. Balancing. Lead Ii Chloride Magnesium Balanced Equation.
From exoahnwtg.blob.core.windows.net
Lead Hydroxide And Hydrogen Chloride at Patsy Hines blog Lead Ii Chloride Magnesium Balanced Equation Balancing chemical equations write a balanced equation for the reaction of molecular nitrogen (n 2) and oxygen (o 2) to form dinitrogen pentoxide. Ionic compounds do not exist as molecules. The balanced equation will be calculated along with the solubility states,. Enter an equation of an ionic chemical equation and press the balance button. \[\ce{pb(no3)2(aq) + nacl(aq) → nano3(aq) +. Lead Ii Chloride Magnesium Balanced Equation.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW Lead Ii Chloride Magnesium Balanced Equation Ionic compounds do not exist as molecules. \[\ce{pb(no3)2(aq) + nacl(aq) → nano3(aq) + pbcl2(s)} \nonumber \] 2. Balancing chemical equations write a balanced equation for the reaction of molecular nitrogen (n 2) and oxygen (o 2) to form dinitrogen pentoxide. The balanced equation will be calculated along with the solubility states,. A solution of lead(ii) nitrate is mixed. \[\ce{pb(no3)2(aq) +. Lead Ii Chloride Magnesium Balanced Equation.
From cerzmopv.blob.core.windows.net
Magnesium And Zinc Nitrate Equation at Terry Ranson blog Lead Ii Chloride Magnesium Balanced Equation \[\ce{pb(no3)2(aq) + nacl(aq) → nano3(aq) + pbcl2(s)} \nonumber \] 2. In order to balance mg on both sides we:. \[\ce{pb(no3)2(aq) + nacl(aq) → nano3(aq) + pbcl2(s)} \nonumber \] 2. 1 atom in reagents and 2 atoms in products. The most complex substance is lead (ii) chloride. 1 mg + 1 pbcl 4 = 1 pb + 2 mgcl 2 mg. Lead Ii Chloride Magnesium Balanced Equation.
From www.youtube.com
Net Ionic Equation for MgSO4 + BaCl2 (Magnesium sulfate and Barium Lead Ii Chloride Magnesium Balanced Equation \[\ce{pb(no3)2(aq) + nacl(aq) → nano3(aq) + pbcl2(s)} \nonumber \] 2. In the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and. In order to balance mg on both sides we:. Balance any equation or reaction using this chemical equation balancer! Ionic compounds do not exist as molecules. Find out what type of reaction. Lead Ii Chloride Magnesium Balanced Equation.
From www.numerade.com
SOLVED Consider the reaction between magnesium nitride and water where Lead Ii Chloride Magnesium Balanced Equation In order to balance mg on both sides we:. Balancing chemical equations write a balanced equation for the reaction of molecular nitrogen (n 2) and oxygen (o 2) to form dinitrogen pentoxide. \[\ce{pb(no3)2(aq) + nacl(aq) → nano3(aq) + pbcl2(s)} \nonumber \] 2. Ionic compounds do not exist as molecules. Enter an equation of an ionic chemical equation and press the. Lead Ii Chloride Magnesium Balanced Equation.
From socratic.org
What is the molecular and net ionic equation for the reaction between Lead Ii Chloride Magnesium Balanced Equation In the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and. The most complex substance is lead (ii) chloride. There are twice as many chloride ions in. A solution of lead(ii) nitrate is mixed. Find out what type of reaction occured. The balanced equation will be calculated along with the solubility states,. \[\ce{pb(no3)2(aq). Lead Ii Chloride Magnesium Balanced Equation.
From www.toppr.com
State your observations when(i) Dilute hydrochloric acid is added to Lead Ii Chloride Magnesium Balanced Equation A solution of lead(ii) nitrate is mixed. \[\ce{pb(no3)2(aq) + nacl(aq) → nano3(aq) + pbcl2(s)} \nonumber \] 2. Balancing chemical equations write a balanced equation for the reaction of molecular nitrogen (n 2) and oxygen (o 2) to form dinitrogen pentoxide. There are twice as many chloride ions in. In order to balance mg on both sides we:. Enter an equation. Lead Ii Chloride Magnesium Balanced Equation.
From www.numerade.com
SOLVED*4.84. Complete and balance the molecular equations for the Lead Ii Chloride Magnesium Balanced Equation Balancing chemical equations write a balanced equation for the reaction of molecular nitrogen (n 2) and oxygen (o 2) to form dinitrogen pentoxide. In order to balance mg on both sides we:. A solution of lead(ii) nitrate is mixed. Ionic compounds do not exist as molecules. There are twice as many chloride ions in. The most complex substance is lead. Lead Ii Chloride Magnesium Balanced Equation.
From www.numerade.com
SOLVED Write the chemical equation for the reaction of magnesium with Lead Ii Chloride Magnesium Balanced Equation The most complex substance is lead (ii) chloride. 1 mg + 1 pbcl 4 = 1 pb + 2 mgcl 2 mg is not balanced: The most complex substance is lead (ii) chloride. A solution of lead(ii) nitrate is mixed. There are twice as many chloride ions in. In order to balance mg on both sides we:. 1 atom in. Lead Ii Chloride Magnesium Balanced Equation.
From www.numerade.com
SOLVED (a) Write the balanced chemical equation following reaction. 1 Lead Ii Chloride Magnesium Balanced Equation \[\ce{pb(no3)2(aq) + nacl(aq) → nano3(aq) + pbcl2(s)} \nonumber \] 2. 1 mg + 1 pbcl 4 = 1 pb + 2 mgcl 2 mg is not balanced: In order to balance mg on both sides we:. A solution of lead(ii) nitrate is mixed. There are twice as many chloride ions in. The balanced equation will be calculated along with the. Lead Ii Chloride Magnesium Balanced Equation.
From www.slideserve.com
PPT Balancing Equations ANSWER KEY PowerPoint Presentation ID2276630 Lead Ii Chloride Magnesium Balanced Equation \[\ce{pb(no3)2(aq) + nacl(aq) → nano3(aq) + pbcl2(s)} \nonumber \] 2. In order to balance mg on both sides we:. A solution of lead(ii) nitrate is mixed. The balanced equation will be calculated along with the solubility states,. Ionic compounds do not exist as molecules. 1 mg + 1 pbcl 4 = 1 pb + 2 mgcl 2 mg is not. Lead Ii Chloride Magnesium Balanced Equation.
From www.slideshare.net
2002 1 Lead Ii Chloride Magnesium Balanced Equation In the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and. A solution of lead(ii) nitrate is mixed. The most complex substance is lead (ii) chloride. Ionic compounds do not exist as molecules. The balanced equation will be calculated along with the solubility states,. There are twice as many chloride ions in. 1. Lead Ii Chloride Magnesium Balanced Equation.
From hydrogenchloridemekaiga.blogspot.com
Hydrogen Chloride Hydrogen Chloride Word Equation Lead Ii Chloride Magnesium Balanced Equation Ionic compounds do not exist as molecules. \[\ce{pb(no3)2(aq) + nacl(aq) → nano3(aq) + pbcl2(s)} \nonumber \] 2. Balance any equation or reaction using this chemical equation balancer! 1 mg + 1 pbcl 4 = 1 pb + 2 mgcl 2 mg is not balanced: Enter an equation of an ionic chemical equation and press the balance button. In order to. Lead Ii Chloride Magnesium Balanced Equation.
From www.youtube.com
How to Balance Pb(NO3)2 + Mg = Pb + Mg(NO3)2 Lead (II) nitrate Lead Ii Chloride Magnesium Balanced Equation The most complex substance is lead (ii) chloride. Balancing chemical equations write a balanced equation for the reaction of molecular nitrogen (n 2) and oxygen (o 2) to form dinitrogen pentoxide. In order to balance mg on both sides we:. Find out what type of reaction occured. \[\ce{pb(no3)2(aq) + nacl(aq) → nano3(aq) + pbcl2(s)} \nonumber \] 2. Ionic compounds do. Lead Ii Chloride Magnesium Balanced Equation.
From www.nagwa.com
Question Video Identifying the Equation That Describes What Happens to Lead Ii Chloride Magnesium Balanced Equation \[\ce{pb(no3)2(aq) + nacl(aq) → nano3(aq) + pbcl2(s)} \nonumber \] 2. There are twice as many chloride ions in. Find out what type of reaction occured. A solution of lead(ii) nitrate is mixed. The balanced equation will be calculated along with the solubility states,. Balance any equation or reaction using this chemical equation balancer! In the solid state, ionic compounds are. Lead Ii Chloride Magnesium Balanced Equation.
From www.youtube.com
Draw the Lewis Structure of MgCl2 (magnesium chloride) YouTube Lead Ii Chloride Magnesium Balanced Equation In order to balance mg on both sides we:. \[\ce{pb(no3)2(aq) + nacl(aq) → nano3(aq) + pbcl2(s)} \nonumber \] 2. Enter an equation of an ionic chemical equation and press the balance button. \[\ce{pb(no3)2(aq) + nacl(aq) → nano3(aq) + pbcl2(s)} \nonumber \] 2. 1 mg + 1 pbcl 4 = 1 pb + 2 mgcl 2 mg is not balanced: A. Lead Ii Chloride Magnesium Balanced Equation.
From www.youtube.com
Equation for PbCl2 + H2O Lead (II) chloride + Water YouTube Lead Ii Chloride Magnesium Balanced Equation Balancing chemical equations write a balanced equation for the reaction of molecular nitrogen (n 2) and oxygen (o 2) to form dinitrogen pentoxide. Find out what type of reaction occured. In order to balance mg on both sides we:. The most complex substance is lead (ii) chloride. There are twice as many chloride ions in. In the solid state, ionic. Lead Ii Chloride Magnesium Balanced Equation.
From www.toppr.com
Two metals which will displace hydrogen and two metals which will not Lead Ii Chloride Magnesium Balanced Equation \[\ce{pb(no3)2(aq) + nacl(aq) → nano3(aq) + pbcl2(s)} \nonumber \] 2. Find out what type of reaction occured. The balanced equation will be calculated along with the solubility states,. There are twice as many chloride ions in. In order to balance mg on both sides we:. In the solid state, ionic compounds are in crystal lattice containing many ions each of. Lead Ii Chloride Magnesium Balanced Equation.
From www.numerade.com
SOLVED Write the balanced COMPLETE ionic equation for the reaction Lead Ii Chloride Magnesium Balanced Equation The most complex substance is lead (ii) chloride. Balance any equation or reaction using this chemical equation balancer! \[\ce{pb(no3)2(aq) + nacl(aq) → nano3(aq) + pbcl2(s)} \nonumber \] 2. Balancing chemical equations write a balanced equation for the reaction of molecular nitrogen (n 2) and oxygen (o 2) to form dinitrogen pentoxide. 1 atom in reagents and 2 atoms in products.. Lead Ii Chloride Magnesium Balanced Equation.
From www.bartleby.com
Answered When aqueous solutions of iron(II)… bartleby Lead Ii Chloride Magnesium Balanced Equation 1 mg + 1 pbcl 4 = 1 pb + 2 mgcl 2 mg is not balanced: \[\ce{pb(no3)2(aq) + nacl(aq) → nano3(aq) + pbcl2(s)} \nonumber \] 2. Enter an equation of an ionic chemical equation and press the balance button. In order to balance mg on both sides we:. \[\ce{pb(no3)2(aq) + nacl(aq) → nano3(aq) + pbcl2(s)} \nonumber \] 2. Ionic. Lead Ii Chloride Magnesium Balanced Equation.
From www.toppr.com
How many moles of lead (II) chloride will be formed from a reaction Lead Ii Chloride Magnesium Balanced Equation The most complex substance is lead (ii) chloride. There are twice as many chloride ions in. Balance any equation or reaction using this chemical equation balancer! Balancing chemical equations write a balanced equation for the reaction of molecular nitrogen (n 2) and oxygen (o 2) to form dinitrogen pentoxide. The balanced equation will be calculated along with the solubility states,.. Lead Ii Chloride Magnesium Balanced Equation.
From astonishingceiyrs.blogspot.com
Magnesium Chloride Formula astonishingceiyrs Lead Ii Chloride Magnesium Balanced Equation Balance any equation or reaction using this chemical equation balancer! \[\ce{pb(no3)2(aq) + nacl(aq) → nano3(aq) + pbcl2(s)} \nonumber \] 2. Ionic compounds do not exist as molecules. Find out what type of reaction occured. The balanced equation will be calculated along with the solubility states,. Balancing chemical equations write a balanced equation for the reaction of molecular nitrogen (n 2). Lead Ii Chloride Magnesium Balanced Equation.
From exoubqalb.blob.core.windows.net
Chloride Formula Is at Carole Dubois blog Lead Ii Chloride Magnesium Balanced Equation In the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and. 1 atom in reagents and 2 atoms in products. Balance any equation or reaction using this chemical equation balancer! Enter an equation of an ionic chemical equation and press the balance button. \[\ce{pb(no3)2(aq) + nacl(aq) → nano3(aq) + pbcl2(s)} \nonumber \] 2.. Lead Ii Chloride Magnesium Balanced Equation.
From www.coursehero.com
[Solved] write the molecular, ionic, and net ionic equation Lead Ii Chloride Magnesium Balanced Equation 1 mg + 1 pbcl 4 = 1 pb + 2 mgcl 2 mg is not balanced: There are twice as many chloride ions in. \[\ce{pb(no3)2(aq) + nacl(aq) → nano3(aq) + pbcl2(s)} \nonumber \] 2. Balancing chemical equations write a balanced equation for the reaction of molecular nitrogen (n 2) and oxygen (o 2) to form dinitrogen pentoxide. Balance any. Lead Ii Chloride Magnesium Balanced Equation.
From www.tessshebaylo.com
Net Ionic Equation Ammonium Bromide Dissolved In Water Tessshebaylo Lead Ii Chloride Magnesium Balanced Equation There are twice as many chloride ions in. 1 mg + 1 pbcl 4 = 1 pb + 2 mgcl 2 mg is not balanced: In order to balance mg on both sides we:. Ionic compounds do not exist as molecules. Balance any equation or reaction using this chemical equation balancer! The balanced equation will be calculated along with the. Lead Ii Chloride Magnesium Balanced Equation.