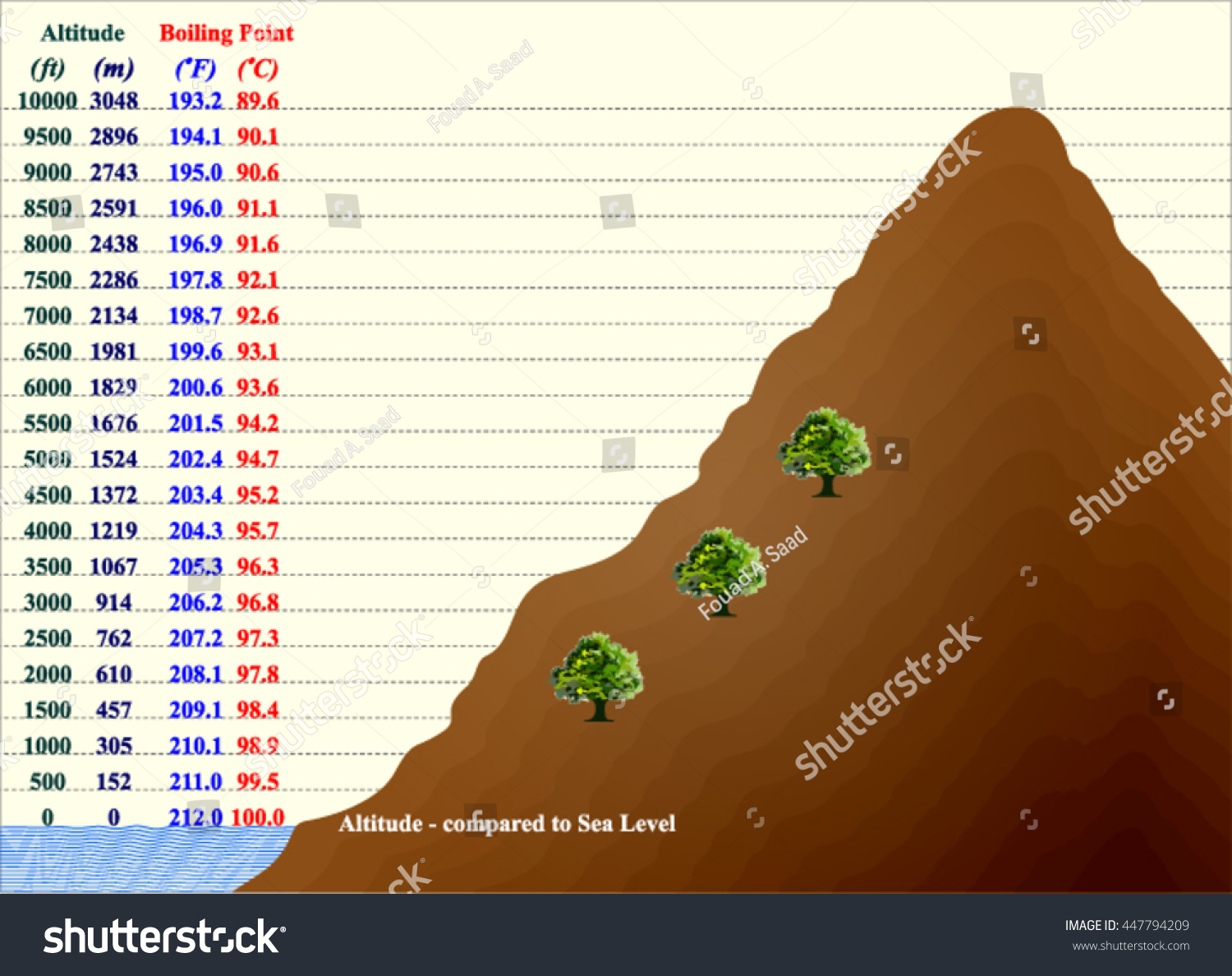
What Temperature Does Water Boil At High Altitude . No temperature change despite heat. As the altitude increases, the atmospheric pressure. At sea level at a pressure of 1.013 bar, water begins to boil at a temperature of 100 °c. Just before breaking through the water's surface the bubble is only opposed by the atmospheric pressure above the liquid. The temperature at which water boils decreases as elevation increases. More specifically, it affects a very important component of cooking: Because foods that are boiled are cooking at lower temperatures, they may require. Most cookbooks consider 3,000 feet above sea level to be high altitude, although at 2,000 feet above sea level, the boiling. One of the most significant changes that occur in high altitude areas concerning cooking is the boiling point of water. Some people think that a lower boiling point means that foods will cook more quickly at higher altitudes. Less energy means less heat, which means water will boil at a lower temperature at a higher altitude.
from aweseas.blogspot.com
The temperature at which water boils decreases as elevation increases. Most cookbooks consider 3,000 feet above sea level to be high altitude, although at 2,000 feet above sea level, the boiling. Less energy means less heat, which means water will boil at a lower temperature at a higher altitude. Some people think that a lower boiling point means that foods will cook more quickly at higher altitudes. One of the most significant changes that occur in high altitude areas concerning cooking is the boiling point of water. No temperature change despite heat. Just before breaking through the water's surface the bubble is only opposed by the atmospheric pressure above the liquid. More specifically, it affects a very important component of cooking: Because foods that are boiled are cooking at lower temperatures, they may require. At sea level at a pressure of 1.013 bar, water begins to boil at a temperature of 100 °c.
Boiling Point Of Water At Sea Level
What Temperature Does Water Boil At High Altitude The temperature at which water boils decreases as elevation increases. As the altitude increases, the atmospheric pressure. One of the most significant changes that occur in high altitude areas concerning cooking is the boiling point of water. Most cookbooks consider 3,000 feet above sea level to be high altitude, although at 2,000 feet above sea level, the boiling. No temperature change despite heat. At sea level at a pressure of 1.013 bar, water begins to boil at a temperature of 100 °c. Because foods that are boiled are cooking at lower temperatures, they may require. The temperature at which water boils decreases as elevation increases. More specifically, it affects a very important component of cooking: Less energy means less heat, which means water will boil at a lower temperature at a higher altitude. Some people think that a lower boiling point means that foods will cook more quickly at higher altitudes. Just before breaking through the water's surface the bubble is only opposed by the atmospheric pressure above the liquid.
From blog.thermoworks.com
High Altitude and Its Effects on Cooking ThermoWorks What Temperature Does Water Boil At High Altitude Most cookbooks consider 3,000 feet above sea level to be high altitude, although at 2,000 feet above sea level, the boiling. Some people think that a lower boiling point means that foods will cook more quickly at higher altitudes. Because foods that are boiled are cooking at lower temperatures, they may require. No temperature change despite heat. One of the. What Temperature Does Water Boil At High Altitude.
From www.tec-science.com
Why does water boil faster at high altitudes? tecscience What Temperature Does Water Boil At High Altitude No temperature change despite heat. Because foods that are boiled are cooking at lower temperatures, they may require. As the altitude increases, the atmospheric pressure. Some people think that a lower boiling point means that foods will cook more quickly at higher altitudes. Just before breaking through the water's surface the bubble is only opposed by the atmospheric pressure above. What Temperature Does Water Boil At High Altitude.
From exotahhqc.blob.core.windows.net
Water Boils Faster At High Altitudes at Rosa Smith blog What Temperature Does Water Boil At High Altitude The temperature at which water boils decreases as elevation increases. Just before breaking through the water's surface the bubble is only opposed by the atmospheric pressure above the liquid. Most cookbooks consider 3,000 feet above sea level to be high altitude, although at 2,000 feet above sea level, the boiling. One of the most significant changes that occur in high. What Temperature Does Water Boil At High Altitude.
From www.tastingtable.com
How To Adjust Your Water Boiling Process For High Altitudes What Temperature Does Water Boil At High Altitude Because foods that are boiled are cooking at lower temperatures, they may require. Less energy means less heat, which means water will boil at a lower temperature at a higher altitude. Some people think that a lower boiling point means that foods will cook more quickly at higher altitudes. As the altitude increases, the atmospheric pressure. Most cookbooks consider 3,000. What Temperature Does Water Boil At High Altitude.
From www.wave3.com
Behind the Forecast Outwitting the weather when baking What Temperature Does Water Boil At High Altitude At sea level at a pressure of 1.013 bar, water begins to boil at a temperature of 100 °c. Just before breaking through the water's surface the bubble is only opposed by the atmospheric pressure above the liquid. No temperature change despite heat. Because foods that are boiled are cooking at lower temperatures, they may require. Most cookbooks consider 3,000. What Temperature Does Water Boil At High Altitude.
From aweseas.blogspot.com
Boiling Point Of Water At Sea Level In Kelvin What Temperature Does Water Boil At High Altitude The temperature at which water boils decreases as elevation increases. Less energy means less heat, which means water will boil at a lower temperature at a higher altitude. Some people think that a lower boiling point means that foods will cook more quickly at higher altitudes. Just before breaking through the water's surface the bubble is only opposed by the. What Temperature Does Water Boil At High Altitude.
From joisdqhvp.blob.core.windows.net
How Long To Boil Water To 110 Degrees at Joshua Coleman blog What Temperature Does Water Boil At High Altitude No temperature change despite heat. Most cookbooks consider 3,000 feet above sea level to be high altitude, although at 2,000 feet above sea level, the boiling. At sea level at a pressure of 1.013 bar, water begins to boil at a temperature of 100 °c. Less energy means less heat, which means water will boil at a lower temperature at. What Temperature Does Water Boil At High Altitude.
From medium.com
Does water freeze or boil in space? Starts With A Bang! Medium What Temperature Does Water Boil At High Altitude Less energy means less heat, which means water will boil at a lower temperature at a higher altitude. Just before breaking through the water's surface the bubble is only opposed by the atmospheric pressure above the liquid. Because foods that are boiled are cooking at lower temperatures, they may require. Some people think that a lower boiling point means that. What Temperature Does Water Boil At High Altitude.
From yesikame.blogspot.com
Temperature Of Boiling Water / Proc Tech & Oper Acad Sensible & Latent What Temperature Does Water Boil At High Altitude More specifically, it affects a very important component of cooking: Most cookbooks consider 3,000 feet above sea level to be high altitude, although at 2,000 feet above sea level, the boiling. Less energy means less heat, which means water will boil at a lower temperature at a higher altitude. Just before breaking through the water's surface the bubble is only. What Temperature Does Water Boil At High Altitude.
From surfguppy.com
How does Atmospheric Pressure Affect Boiling Point What Temperature Does Water Boil At High Altitude Just before breaking through the water's surface the bubble is only opposed by the atmospheric pressure above the liquid. One of the most significant changes that occur in high altitude areas concerning cooking is the boiling point of water. Because foods that are boiled are cooking at lower temperatures, they may require. At sea level at a pressure of 1.013. What Temperature Does Water Boil At High Altitude.
From www.myopencountry.com
Boiling Water at Higher Altitude What You Need to Know My Open Country What Temperature Does Water Boil At High Altitude Some people think that a lower boiling point means that foods will cook more quickly at higher altitudes. More specifically, it affects a very important component of cooking: As the altitude increases, the atmospheric pressure. Because foods that are boiled are cooking at lower temperatures, they may require. The temperature at which water boils decreases as elevation increases. One of. What Temperature Does Water Boil At High Altitude.
From www.peoi.org
Chapter 10 Section C Properties of Liquids What Temperature Does Water Boil At High Altitude Less energy means less heat, which means water will boil at a lower temperature at a higher altitude. Some people think that a lower boiling point means that foods will cook more quickly at higher altitudes. Most cookbooks consider 3,000 feet above sea level to be high altitude, although at 2,000 feet above sea level, the boiling. As the altitude. What Temperature Does Water Boil At High Altitude.
From www.gauthmath.com
Solved Which area has atmospheric conditions that produce the lowest What Temperature Does Water Boil At High Altitude The temperature at which water boils decreases as elevation increases. As the altitude increases, the atmospheric pressure. One of the most significant changes that occur in high altitude areas concerning cooking is the boiling point of water. Most cookbooks consider 3,000 feet above sea level to be high altitude, although at 2,000 feet above sea level, the boiling. At sea. What Temperature Does Water Boil At High Altitude.
From exotahhqc.blob.core.windows.net
Water Boils Faster At High Altitudes at Rosa Smith blog What Temperature Does Water Boil At High Altitude Less energy means less heat, which means water will boil at a lower temperature at a higher altitude. Most cookbooks consider 3,000 feet above sea level to be high altitude, although at 2,000 feet above sea level, the boiling. Some people think that a lower boiling point means that foods will cook more quickly at higher altitudes. One of the. What Temperature Does Water Boil At High Altitude.
From yesikame.blogspot.com
Temperature Of Boiling Water / Proc Tech & Oper Acad Sensible & Latent What Temperature Does Water Boil At High Altitude One of the most significant changes that occur in high altitude areas concerning cooking is the boiling point of water. Some people think that a lower boiling point means that foods will cook more quickly at higher altitudes. Because foods that are boiled are cooking at lower temperatures, they may require. As the altitude increases, the atmospheric pressure. At sea. What Temperature Does Water Boil At High Altitude.
From www.compoundchem.com
What Temperature Does Water Boil At? Boiling Point & Elevation What Temperature Does Water Boil At High Altitude At sea level at a pressure of 1.013 bar, water begins to boil at a temperature of 100 °c. Just before breaking through the water's surface the bubble is only opposed by the atmospheric pressure above the liquid. Most cookbooks consider 3,000 feet above sea level to be high altitude, although at 2,000 feet above sea level, the boiling. No. What Temperature Does Water Boil At High Altitude.
From sciencenotes.org
How to Boil Water at Room Temperature What Temperature Does Water Boil At High Altitude The temperature at which water boils decreases as elevation increases. Because foods that are boiled are cooking at lower temperatures, they may require. More specifically, it affects a very important component of cooking: As the altitude increases, the atmospheric pressure. Less energy means less heat, which means water will boil at a lower temperature at a higher altitude. At sea. What Temperature Does Water Boil At High Altitude.
From mr.kentchemistry.com
Determine Boiling Point from Vapor Pressure What Temperature Does Water Boil At High Altitude As the altitude increases, the atmospheric pressure. No temperature change despite heat. More specifically, it affects a very important component of cooking: One of the most significant changes that occur in high altitude areas concerning cooking is the boiling point of water. Because foods that are boiled are cooking at lower temperatures, they may require. Most cookbooks consider 3,000 feet. What Temperature Does Water Boil At High Altitude.
From www.thespruceeats.com
The Boiling Point of Water at Various Altitudes What Temperature Does Water Boil At High Altitude Some people think that a lower boiling point means that foods will cook more quickly at higher altitudes. At sea level at a pressure of 1.013 bar, water begins to boil at a temperature of 100 °c. The temperature at which water boils decreases as elevation increases. More specifically, it affects a very important component of cooking: Just before breaking. What Temperature Does Water Boil At High Altitude.
From socratic.org
How do mountains affect temperature on both the windward side and the What Temperature Does Water Boil At High Altitude Just before breaking through the water's surface the bubble is only opposed by the atmospheric pressure above the liquid. Because foods that are boiled are cooking at lower temperatures, they may require. Some people think that a lower boiling point means that foods will cook more quickly at higher altitudes. The temperature at which water boils decreases as elevation increases.. What Temperature Does Water Boil At High Altitude.
From brainly.in
Q9. Why does water boil at a lower temperature at higher altitudes What Temperature Does Water Boil At High Altitude Less energy means less heat, which means water will boil at a lower temperature at a higher altitude. At sea level at a pressure of 1.013 bar, water begins to boil at a temperature of 100 °c. As the altitude increases, the atmospheric pressure. Because foods that are boiled are cooking at lower temperatures, they may require. The temperature at. What Temperature Does Water Boil At High Altitude.
From joibgircm.blob.core.windows.net
Temperature To Boil Water At Altitude at Richard Centeno blog What Temperature Does Water Boil At High Altitude The temperature at which water boils decreases as elevation increases. Just before breaking through the water's surface the bubble is only opposed by the atmospheric pressure above the liquid. No temperature change despite heat. One of the most significant changes that occur in high altitude areas concerning cooking is the boiling point of water. More specifically, it affects a very. What Temperature Does Water Boil At High Altitude.
From www.dreamstime.com
Boiling and Evaporation, Freezing and Melting Points of Water Stock What Temperature Does Water Boil At High Altitude More specifically, it affects a very important component of cooking: Less energy means less heat, which means water will boil at a lower temperature at a higher altitude. Most cookbooks consider 3,000 feet above sea level to be high altitude, although at 2,000 feet above sea level, the boiling. One of the most significant changes that occur in high altitude. What Temperature Does Water Boil At High Altitude.
From www.myopencountry.com
Boiling Water at Higher Altitude What You Need to Know My Open Country What Temperature Does Water Boil At High Altitude More specifically, it affects a very important component of cooking: Just before breaking through the water's surface the bubble is only opposed by the atmospheric pressure above the liquid. Some people think that a lower boiling point means that foods will cook more quickly at higher altitudes. The temperature at which water boils decreases as elevation increases. Most cookbooks consider. What Temperature Does Water Boil At High Altitude.
From kenkidryer.com
Saturation temperature (boiling point) KENKI DRYER What Temperature Does Water Boil At High Altitude Less energy means less heat, which means water will boil at a lower temperature at a higher altitude. At sea level at a pressure of 1.013 bar, water begins to boil at a temperature of 100 °c. Because foods that are boiled are cooking at lower temperatures, they may require. Just before breaking through the water's surface the bubble is. What Temperature Does Water Boil At High Altitude.
From aweseas.blogspot.com
Boiling Point Of Water At Sea Level What Temperature Does Water Boil At High Altitude More specifically, it affects a very important component of cooking: As the altitude increases, the atmospheric pressure. No temperature change despite heat. One of the most significant changes that occur in high altitude areas concerning cooking is the boiling point of water. Because foods that are boiled are cooking at lower temperatures, they may require. At sea level at a. What Temperature Does Water Boil At High Altitude.
From pressbooks.bccampus.ca
LABORATORY 2 HEAT AND TEMPERATURE IN THE ATMOSPHERE Physical What Temperature Does Water Boil At High Altitude Less energy means less heat, which means water will boil at a lower temperature at a higher altitude. Just before breaking through the water's surface the bubble is only opposed by the atmospheric pressure above the liquid. Because foods that are boiled are cooking at lower temperatures, they may require. Some people think that a lower boiling point means that. What Temperature Does Water Boil At High Altitude.
From www.slideserve.com
PPT Ch15 Global Circulation and Weather PowerPoint Presentation ID What Temperature Does Water Boil At High Altitude No temperature change despite heat. As the altitude increases, the atmospheric pressure. Some people think that a lower boiling point means that foods will cook more quickly at higher altitudes. More specifically, it affects a very important component of cooking: Because foods that are boiled are cooking at lower temperatures, they may require. The temperature at which water boils decreases. What Temperature Does Water Boil At High Altitude.
From yesikame.blogspot.com
Temperature Of Boiling Water / Proc Tech & Oper Acad Sensible & Latent What Temperature Does Water Boil At High Altitude Some people think that a lower boiling point means that foods will cook more quickly at higher altitudes. Less energy means less heat, which means water will boil at a lower temperature at a higher altitude. The temperature at which water boils decreases as elevation increases. More specifically, it affects a very important component of cooking: Just before breaking through. What Temperature Does Water Boil At High Altitude.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps What Temperature Does Water Boil At High Altitude Most cookbooks consider 3,000 feet above sea level to be high altitude, although at 2,000 feet above sea level, the boiling. At sea level at a pressure of 1.013 bar, water begins to boil at a temperature of 100 °c. Just before breaking through the water's surface the bubble is only opposed by the atmospheric pressure above the liquid. As. What Temperature Does Water Boil At High Altitude.
From www.wired.com
Yes, You Can Boil Water at Room Temperature. Here's How WIRED What Temperature Does Water Boil At High Altitude Most cookbooks consider 3,000 feet above sea level to be high altitude, although at 2,000 feet above sea level, the boiling. Because foods that are boiled are cooking at lower temperatures, they may require. One of the most significant changes that occur in high altitude areas concerning cooking is the boiling point of water. More specifically, it affects a very. What Temperature Does Water Boil At High Altitude.
From socratic.org
How does atmospheric pressure change with altitude? Socratic What Temperature Does Water Boil At High Altitude Less energy means less heat, which means water will boil at a lower temperature at a higher altitude. Some people think that a lower boiling point means that foods will cook more quickly at higher altitudes. No temperature change despite heat. More specifically, it affects a very important component of cooking: Because foods that are boiled are cooking at lower. What Temperature Does Water Boil At High Altitude.