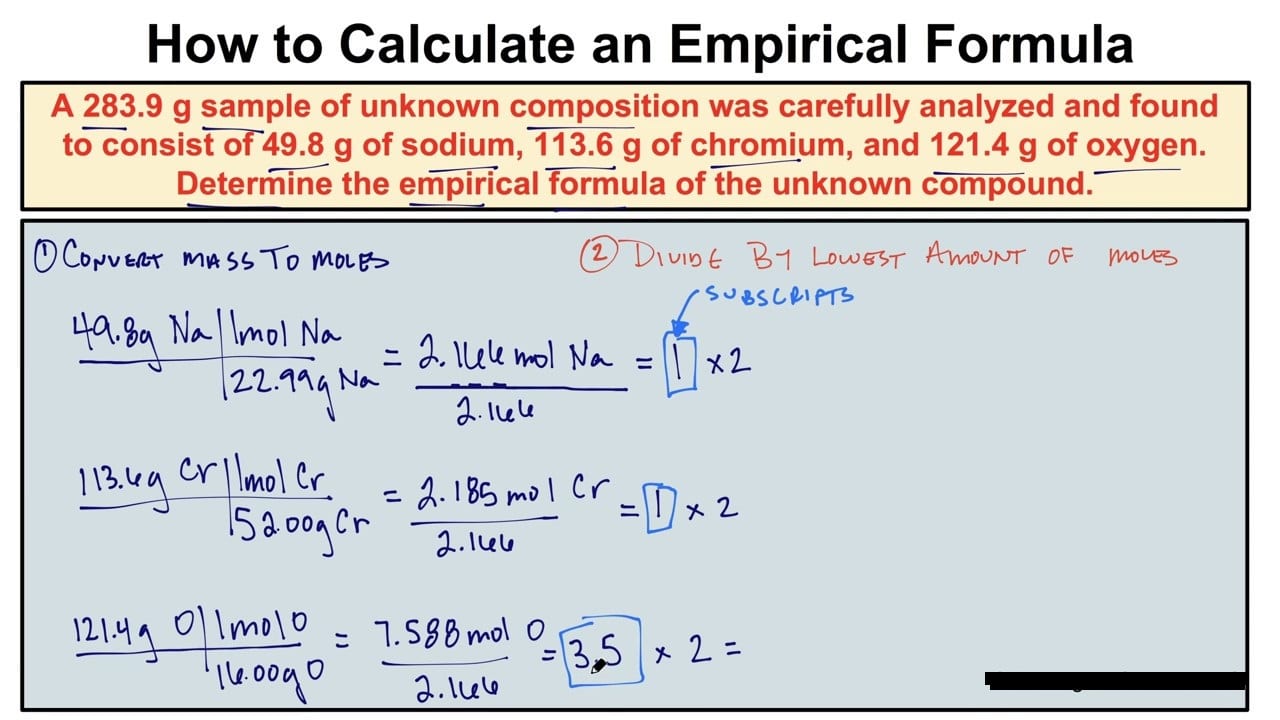
Calcium Chloride Empirical Formula . Therefore, a sample of calcium chloride contains twice as many. The previous section discussed the relationship. Calculating the empirical formula from a reaction. Determine the molecular formula of a compound. Determine the empirical formula of a compound. Ga 3 + and as 3 −; Yes, it's an empirical formula. The subscripts are whole numbers and. The empirical formula of the compound is \(\ce{fe_2o_3}\). Write the empirical formula for the simplest binary ionic compound formed from each ion or element pair. If the salt is in its. 20 g of calcium was found to react with 35·5 g of chlorine. Calcium chloride is an ionic compound with the chemical formula cacl2.
from geteducationbee.com
The empirical formula of the compound is \(\ce{fe_2o_3}\). Yes, it's an empirical formula. The previous section discussed the relationship. Determine the molecular formula of a compound. Determine the empirical formula of a compound. Write the empirical formula for the simplest binary ionic compound formed from each ion or element pair. The subscripts are whole numbers and. Calculating the empirical formula from a reaction. Calcium chloride is an ionic compound with the chemical formula cacl2. Therefore, a sample of calcium chloride contains twice as many.
Empirical Formula Definition, Calculator, Best Examples Get Education Bee
Calcium Chloride Empirical Formula Determine the empirical formula of a compound. If the salt is in its. Determine the molecular formula of a compound. Calculating the empirical formula from a reaction. Write the empirical formula for the simplest binary ionic compound formed from each ion or element pair. The subscripts are whole numbers and. Calcium chloride is an ionic compound with the chemical formula cacl2. The previous section discussed the relationship. Determine the empirical formula of a compound. Yes, it's an empirical formula. Therefore, a sample of calcium chloride contains twice as many. 20 g of calcium was found to react with 35·5 g of chlorine. Ga 3 + and as 3 −; The empirical formula of the compound is \(\ce{fe_2o_3}\).
From slideplayer.com
Elements and Compounds Chapter 3 ppt download Calcium Chloride Empirical Formula Determine the molecular formula of a compound. Ga 3 + and as 3 −; The previous section discussed the relationship. Determine the empirical formula of a compound. Therefore, a sample of calcium chloride contains twice as many. Calculating the empirical formula from a reaction. Yes, it's an empirical formula. If the salt is in its. Write the empirical formula for. Calcium Chloride Empirical Formula.
From pametno21.blogspot.com
1 Formula Unit Of Calcium Chloride pametno Calcium Chloride Empirical Formula Yes, it's an empirical formula. The subscripts are whole numbers and. Determine the empirical formula of a compound. Write the empirical formula for the simplest binary ionic compound formed from each ion or element pair. Calcium chloride is an ionic compound with the chemical formula cacl2. Calculating the empirical formula from a reaction. The empirical formula of the compound is. Calcium Chloride Empirical Formula.
From www.numerade.com
SOLVED Experiment Determination of an Empirical Formula Report Sheet Calcium Chloride Empirical Formula The previous section discussed the relationship. Write the empirical formula for the simplest binary ionic compound formed from each ion or element pair. Determine the empirical formula of a compound. Calculating the empirical formula from a reaction. Yes, it's an empirical formula. 20 g of calcium was found to react with 35·5 g of chlorine. Therefore, a sample of calcium. Calcium Chloride Empirical Formula.
From www.youtube.com
How to Write the Net Ionic Equation for CaCl2 + Na2CO3 = CaCO3 + NaCl Calcium Chloride Empirical Formula Yes, it's an empirical formula. The previous section discussed the relationship. Calcium chloride is an ionic compound with the chemical formula cacl2. Write the empirical formula for the simplest binary ionic compound formed from each ion or element pair. Determine the empirical formula of a compound. Therefore, a sample of calcium chloride contains twice as many. 20 g of calcium. Calcium Chloride Empirical Formula.
From formulasense.com
What is Calcium Chloride? — Formula Sense Calcium Chloride Empirical Formula 20 g of calcium was found to react with 35·5 g of chlorine. If the salt is in its. Calculating the empirical formula from a reaction. Determine the molecular formula of a compound. Determine the empirical formula of a compound. Yes, it's an empirical formula. The previous section discussed the relationship. Write the empirical formula for the simplest binary ionic. Calcium Chloride Empirical Formula.
From www.meritnation.com
WRITE THE CHEMICAL FORMULA FOR CALCIUM CHLORIDE AND SODIUM SULPHATE Calcium Chloride Empirical Formula Write the empirical formula for the simplest binary ionic compound formed from each ion or element pair. The empirical formula of the compound is \(\ce{fe_2o_3}\). The subscripts are whole numbers and. Ga 3 + and as 3 −; Yes, it's an empirical formula. Determine the empirical formula of a compound. Determine the molecular formula of a compound. Calcium chloride is. Calcium Chloride Empirical Formula.
From www.tessshebaylo.com
Balanced Chemical Equation For The Dissolution Of Calcium Chloride In Calcium Chloride Empirical Formula If the salt is in its. Therefore, a sample of calcium chloride contains twice as many. Ga 3 + and as 3 −; The empirical formula of the compound is \(\ce{fe_2o_3}\). 20 g of calcium was found to react with 35·5 g of chlorine. The subscripts are whole numbers and. The previous section discussed the relationship. Determine the empirical formula. Calcium Chloride Empirical Formula.
From geteducationbee.com
Empirical Formula Definition, Calculator, Best Examples Get Education Bee Calcium Chloride Empirical Formula The empirical formula of the compound is \(\ce{fe_2o_3}\). Ga 3 + and as 3 −; Yes, it's an empirical formula. Determine the molecular formula of a compound. The subscripts are whole numbers and. Write the empirical formula for the simplest binary ionic compound formed from each ion or element pair. Calcium chloride is an ionic compound with the chemical formula. Calcium Chloride Empirical Formula.
From stock.adobe.com
Molecular formula of calcium chloride. Chemical structure of calcium Calcium Chloride Empirical Formula Write the empirical formula for the simplest binary ionic compound formed from each ion or element pair. If the salt is in its. The subscripts are whole numbers and. Calcium chloride is an ionic compound with the chemical formula cacl2. Calculating the empirical formula from a reaction. Ga 3 + and as 3 −; Yes, it's an empirical formula. Determine. Calcium Chloride Empirical Formula.
From www.youtube.com
How to Write the Formula for Calcium chlorite YouTube Calcium Chloride Empirical Formula Write the empirical formula for the simplest binary ionic compound formed from each ion or element pair. The previous section discussed the relationship. The subscripts are whole numbers and. The empirical formula of the compound is \(\ce{fe_2o_3}\). If the salt is in its. 20 g of calcium was found to react with 35·5 g of chlorine. Determine the empirical formula. Calcium Chloride Empirical Formula.
From www.pw.live
Calcium Chloride Formula, Structure, Properties, Uses Calcium Chloride Empirical Formula The empirical formula of the compound is \(\ce{fe_2o_3}\). Calculating the empirical formula from a reaction. 20 g of calcium was found to react with 35·5 g of chlorine. Determine the molecular formula of a compound. Therefore, a sample of calcium chloride contains twice as many. The previous section discussed the relationship. Ga 3 + and as 3 −; Yes, it's. Calcium Chloride Empirical Formula.
From www.slideserve.com
PPT Naming ionic compounds PowerPoint Presentation, free download Calcium Chloride Empirical Formula Calcium chloride is an ionic compound with the chemical formula cacl2. The previous section discussed the relationship. The subscripts are whole numbers and. Determine the molecular formula of a compound. 20 g of calcium was found to react with 35·5 g of chlorine. Calculating the empirical formula from a reaction. Ga 3 + and as 3 −; The empirical formula. Calcium Chloride Empirical Formula.
From testbook.com
Calcium chloride formula Structure, Molecular Formula & Uses Calcium Chloride Empirical Formula 20 g of calcium was found to react with 35·5 g of chlorine. Therefore, a sample of calcium chloride contains twice as many. The previous section discussed the relationship. Yes, it's an empirical formula. Calculating the empirical formula from a reaction. Calcium chloride is an ionic compound with the chemical formula cacl2. Determine the empirical formula of a compound. Determine. Calcium Chloride Empirical Formula.
From www.youtube.com
Write the chemical formula of the following compounds (a)Magnesium Calcium Chloride Empirical Formula Calculating the empirical formula from a reaction. The subscripts are whole numbers and. Calcium chloride is an ionic compound with the chemical formula cacl2. Write the empirical formula for the simplest binary ionic compound formed from each ion or element pair. If the salt is in its. Determine the empirical formula of a compound. Ga 3 + and as 3. Calcium Chloride Empirical Formula.
From sharedocnow.blogspot.com
Formula For Calcium Chloride sharedoc Calcium Chloride Empirical Formula Determine the empirical formula of a compound. Yes, it's an empirical formula. 20 g of calcium was found to react with 35·5 g of chlorine. The subscripts are whole numbers and. If the salt is in its. Calculating the empirical formula from a reaction. The empirical formula of the compound is \(\ce{fe_2o_3}\). Calcium chloride is an ionic compound with the. Calcium Chloride Empirical Formula.
From www.numerade.com
SOLVED Calcium chloride, CaCl2, is a strong electrolyte with a formula Calcium Chloride Empirical Formula Ga 3 + and as 3 −; The previous section discussed the relationship. If the salt is in its. Determine the molecular formula of a compound. The subscripts are whole numbers and. Determine the empirical formula of a compound. Write the empirical formula for the simplest binary ionic compound formed from each ion or element pair. The empirical formula of. Calcium Chloride Empirical Formula.
From www.dreamstime.com
Molecular Formula of Calcium Chloride Stock Illustration Illustration Calcium Chloride Empirical Formula Write the empirical formula for the simplest binary ionic compound formed from each ion or element pair. The subscripts are whole numbers and. Yes, it's an empirical formula. Determine the molecular formula of a compound. If the salt is in its. Calcium chloride is an ionic compound with the chemical formula cacl2. Calculating the empirical formula from a reaction. 20. Calcium Chloride Empirical Formula.
From www.slideserve.com
PPT Calcium Chloride PowerPoint Presentation, free download ID2156417 Calcium Chloride Empirical Formula Determine the molecular formula of a compound. The previous section discussed the relationship. The empirical formula of the compound is \(\ce{fe_2o_3}\). Yes, it's an empirical formula. Write the empirical formula for the simplest binary ionic compound formed from each ion or element pair. 20 g of calcium was found to react with 35·5 g of chlorine. If the salt is. Calcium Chloride Empirical Formula.
From molekula.com
Purchase Calcium chloride anhydrous [10043524] online • Catalog Calcium Chloride Empirical Formula The subscripts are whole numbers and. The empirical formula of the compound is \(\ce{fe_2o_3}\). Determine the empirical formula of a compound. The previous section discussed the relationship. Calculating the empirical formula from a reaction. Ga 3 + and as 3 −; Determine the molecular formula of a compound. Calcium chloride is an ionic compound with the chemical formula cacl2. If. Calcium Chloride Empirical Formula.
From mavink.com
Calcium Chloride Symbol Calcium Chloride Empirical Formula Determine the empirical formula of a compound. Write the empirical formula for the simplest binary ionic compound formed from each ion or element pair. Ga 3 + and as 3 −; The subscripts are whole numbers and. The empirical formula of the compound is \(\ce{fe_2o_3}\). Determine the molecular formula of a compound. If the salt is in its. Yes, it's. Calcium Chloride Empirical Formula.
From www.numerade.com
SOLVEDIf 2.461 g of metallic calcium is heated in a stream of chlorine Calcium Chloride Empirical Formula Yes, it's an empirical formula. Determine the molecular formula of a compound. Calculating the empirical formula from a reaction. If the salt is in its. The empirical formula of the compound is \(\ce{fe_2o_3}\). The subscripts are whole numbers and. Write the empirical formula for the simplest binary ionic compound formed from each ion or element pair. Therefore, a sample of. Calcium Chloride Empirical Formula.
From www.youtube.com
CaCl2 Lewis Structure How to draw the Lewis Dot Structure for Calcium Calcium Chloride Empirical Formula Write the empirical formula for the simplest binary ionic compound formed from each ion or element pair. Therefore, a sample of calcium chloride contains twice as many. 20 g of calcium was found to react with 35·5 g of chlorine. Calcium chloride is an ionic compound with the chemical formula cacl2. The previous section discussed the relationship. Calculating the empirical. Calcium Chloride Empirical Formula.
From studylib.net
empirical formula Calcium Chloride Empirical Formula Yes, it's an empirical formula. Ga 3 + and as 3 −; Determine the empirical formula of a compound. The subscripts are whole numbers and. Calculating the empirical formula from a reaction. The previous section discussed the relationship. Determine the molecular formula of a compound. 20 g of calcium was found to react with 35·5 g of chlorine. Therefore, a. Calcium Chloride Empirical Formula.
From www.coursehero.com
[Solved] . 3. Using the Lewis structures of calcium chloride and Calcium Chloride Empirical Formula Therefore, a sample of calcium chloride contains twice as many. Calcium chloride is an ionic compound with the chemical formula cacl2. Ga 3 + and as 3 −; The previous section discussed the relationship. 20 g of calcium was found to react with 35·5 g of chlorine. Calculating the empirical formula from a reaction. Determine the molecular formula of a. Calcium Chloride Empirical Formula.
From studylib.net
Ionic Compounds Naming Calcium Chloride Empirical Formula The subscripts are whole numbers and. The empirical formula of the compound is \(\ce{fe_2o_3}\). Calcium chloride is an ionic compound with the chemical formula cacl2. Therefore, a sample of calcium chloride contains twice as many. Ga 3 + and as 3 −; Determine the empirical formula of a compound. Calculating the empirical formula from a reaction. Write the empirical formula. Calcium Chloride Empirical Formula.
From www.youtube.com
Calcium chloride YouTube Calcium Chloride Empirical Formula 20 g of calcium was found to react with 35·5 g of chlorine. If the salt is in its. Determine the empirical formula of a compound. Write the empirical formula for the simplest binary ionic compound formed from each ion or element pair. Therefore, a sample of calcium chloride contains twice as many. Determine the molecular formula of a compound.. Calcium Chloride Empirical Formula.
From www.slideserve.com
PPT CHEMISTRY PowerPoint Presentation, free download ID9471742 Calcium Chloride Empirical Formula Ga 3 + and as 3 −; Calculating the empirical formula from a reaction. The subscripts are whole numbers and. Determine the molecular formula of a compound. The previous section discussed the relationship. If the salt is in its. Therefore, a sample of calcium chloride contains twice as many. Yes, it's an empirical formula. Determine the empirical formula of a. Calcium Chloride Empirical Formula.
From molekula.com
Purchase Calcium chloride dihydrate [10035048] online • Catalog Calcium Chloride Empirical Formula Determine the molecular formula of a compound. The empirical formula of the compound is \(\ce{fe_2o_3}\). Determine the empirical formula of a compound. Write the empirical formula for the simplest binary ionic compound formed from each ion or element pair. Calculating the empirical formula from a reaction. The previous section discussed the relationship. Ga 3 + and as 3 −; The. Calcium Chloride Empirical Formula.
From www.chegg.com
Complete the table below by deciding whether aqueous Calcium Chloride Empirical Formula Yes, it's an empirical formula. If the salt is in its. Calculating the empirical formula from a reaction. The subscripts are whole numbers and. Determine the molecular formula of a compound. Therefore, a sample of calcium chloride contains twice as many. Determine the empirical formula of a compound. Ga 3 + and as 3 −; 20 g of calcium was. Calcium Chloride Empirical Formula.
From slideplayer.com
Ch. 5 Ionic Compounds. ppt download Calcium Chloride Empirical Formula Yes, it's an empirical formula. The subscripts are whole numbers and. Write the empirical formula for the simplest binary ionic compound formed from each ion or element pair. Calcium chloride is an ionic compound with the chemical formula cacl2. Determine the empirical formula of a compound. Therefore, a sample of calcium chloride contains twice as many. 20 g of calcium. Calcium Chloride Empirical Formula.
From www.tessshebaylo.com
Chemical Equation For Water And Calcium Oxide Tessshebaylo Calcium Chloride Empirical Formula Determine the molecular formula of a compound. Yes, it's an empirical formula. Therefore, a sample of calcium chloride contains twice as many. 20 g of calcium was found to react with 35·5 g of chlorine. Determine the empirical formula of a compound. Write the empirical formula for the simplest binary ionic compound formed from each ion or element pair. The. Calcium Chloride Empirical Formula.
From www.numerade.com
SOLVEDIf 2.461 g of metallic calcium is heated in a stream of chlorine Calcium Chloride Empirical Formula Determine the empirical formula of a compound. 20 g of calcium was found to react with 35·5 g of chlorine. Yes, it's an empirical formula. Therefore, a sample of calcium chloride contains twice as many. The previous section discussed the relationship. Calcium chloride is an ionic compound with the chemical formula cacl2. The empirical formula of the compound is \(\ce{fe_2o_3}\).. Calcium Chloride Empirical Formula.
From revolutionfermentation.com
What Is Calcium Chloride And How to Use It? Revolution Fermentation Calcium Chloride Empirical Formula Yes, it's an empirical formula. The empirical formula of the compound is \(\ce{fe_2o_3}\). 20 g of calcium was found to react with 35·5 g of chlorine. Calculating the empirical formula from a reaction. If the salt is in its. Therefore, a sample of calcium chloride contains twice as many. Determine the molecular formula of a compound. The subscripts are whole. Calcium Chloride Empirical Formula.
From www.shutterstock.com
Calcium Chloride Molecular Structure Formula Periodic Stock Vector Calcium Chloride Empirical Formula Yes, it's an empirical formula. Determine the molecular formula of a compound. Determine the empirical formula of a compound. 20 g of calcium was found to react with 35·5 g of chlorine. Calcium chloride is an ionic compound with the chemical formula cacl2. If the salt is in its. The subscripts are whole numbers and. Therefore, a sample of calcium. Calcium Chloride Empirical Formula.
From stock.adobe.com
Calcium Chloride CaCl2 molecule. Simple molecular formula consisting of Calcium Chloride Empirical Formula Determine the empirical formula of a compound. If the salt is in its. Determine the molecular formula of a compound. Yes, it's an empirical formula. 20 g of calcium was found to react with 35·5 g of chlorine. Calculating the empirical formula from a reaction. The previous section discussed the relationship. Calcium chloride is an ionic compound with the chemical. Calcium Chloride Empirical Formula.