Iron Iii Oxide And Hydrochloric Acid . Iron(iii) oxide reacts with acids, such as hydrochloric acid, to form iron(iii) chloride and water. Fe2o3 + hcl = fecl3 + h2o is a double displacement (metathesis) reaction where one mole of hematite [fe 2 o 3] and six moles of. Iron(iii) oxide is also insoluble in water and. Chemical properties of iron(iii) oxide reaction with acids. 6hcl (aq) + fe2o3 (s) —+3h20 (1) + 2fecl3 (aq) we can interpret this to mean: Iron(iii) oxide is soluble in strong acids such as hydrochloric acid (hcl) and sulfuric acid (h 2 so 4). The iron(iii) oxide and sulfuric acid react together under hot conditions and produce the salt iron(iii) sulfate as well. Iron(ii) oxide is a black solid that is insoluble in water and is easily oxidized by hot air. This reaction demonstrates its basic nature. Fe₂o₃ + 6𝐻𝐶𝑙 → 2𝐹𝑒𝐶𝑙₃ + 3𝐻₂𝑂; Now that we have discussed the various highlights of iron(iii) oxide we. Enter an equation of an ionic chemical equation and press the balance button. The balanced chemical equation for the reaction between hydrochloric acid and iron (iii) oxide is: The balanced equation will be calculated along with the.
from www.chegg.com
The balanced chemical equation for the reaction between hydrochloric acid and iron (iii) oxide is: Iron(iii) oxide is soluble in strong acids such as hydrochloric acid (hcl) and sulfuric acid (h 2 so 4). This reaction demonstrates its basic nature. Enter an equation of an ionic chemical equation and press the balance button. Iron(iii) oxide reacts with acids, such as hydrochloric acid, to form iron(iii) chloride and water. Chemical properties of iron(iii) oxide reaction with acids. Iron(ii) oxide is a black solid that is insoluble in water and is easily oxidized by hot air. Iron(iii) oxide is also insoluble in water and. Fe2o3 + hcl = fecl3 + h2o is a double displacement (metathesis) reaction where one mole of hematite [fe 2 o 3] and six moles of. Now that we have discussed the various highlights of iron(iii) oxide we.
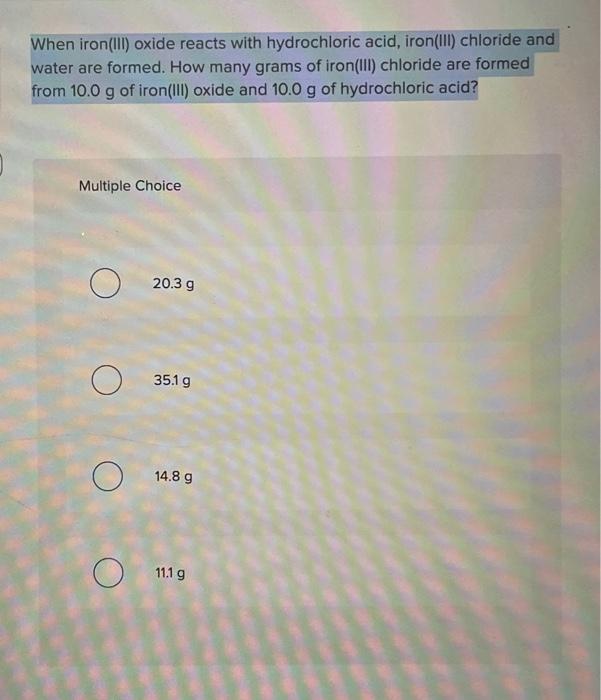
Solved When iron(III) oxide reacts with hydrochloric acid,
Iron Iii Oxide And Hydrochloric Acid Now that we have discussed the various highlights of iron(iii) oxide we. This reaction demonstrates its basic nature. The iron(iii) oxide and sulfuric acid react together under hot conditions and produce the salt iron(iii) sulfate as well. Iron(iii) oxide reacts with acids, such as hydrochloric acid, to form iron(iii) chloride and water. 6hcl (aq) + fe2o3 (s) —+3h20 (1) + 2fecl3 (aq) we can interpret this to mean: Now that we have discussed the various highlights of iron(iii) oxide we. Fe₂o₃ + 6𝐻𝐶𝑙 → 2𝐹𝑒𝐶𝑙₃ + 3𝐻₂𝑂; The balanced chemical equation for the reaction between hydrochloric acid and iron (iii) oxide is: Enter an equation of an ionic chemical equation and press the balance button. Iron(iii) oxide is also insoluble in water and. Fe2o3 + hcl = fecl3 + h2o is a double displacement (metathesis) reaction where one mole of hematite [fe 2 o 3] and six moles of. Iron(iii) oxide is soluble in strong acids such as hydrochloric acid (hcl) and sulfuric acid (h 2 so 4). Chemical properties of iron(iii) oxide reaction with acids. Iron(ii) oxide is a black solid that is insoluble in water and is easily oxidized by hot air. The balanced equation will be calculated along with the.
From www.slideshare.net
Metals Reactivity Series Iron Iii Oxide And Hydrochloric Acid Fe₂o₃ + 6𝐻𝐶𝑙 → 2𝐹𝑒𝐶𝑙₃ + 3𝐻₂𝑂; Iron(iii) oxide reacts with acids, such as hydrochloric acid, to form iron(iii) chloride and water. Fe2o3 + hcl = fecl3 + h2o is a double displacement (metathesis) reaction where one mole of hematite [fe 2 o 3] and six moles of. Iron(iii) oxide is soluble in strong acids such as hydrochloric acid (hcl). Iron Iii Oxide And Hydrochloric Acid.
From slideplayer.com
Connect 14 November, 2018 Metal Oxides Key Words Oxide Acid Alkali Iron Iii Oxide And Hydrochloric Acid 6hcl (aq) + fe2o3 (s) —+3h20 (1) + 2fecl3 (aq) we can interpret this to mean: Iron(ii) oxide is a black solid that is insoluble in water and is easily oxidized by hot air. The balanced chemical equation for the reaction between hydrochloric acid and iron (iii) oxide is: The iron(iii) oxide and sulfuric acid react together under hot conditions. Iron Iii Oxide And Hydrochloric Acid.
From www.youtube.com
Iron Reaction With Hydrochloric Acid YouTube Iron Iii Oxide And Hydrochloric Acid Enter an equation of an ionic chemical equation and press the balance button. 6hcl (aq) + fe2o3 (s) —+3h20 (1) + 2fecl3 (aq) we can interpret this to mean: Iron(ii) oxide is a black solid that is insoluble in water and is easily oxidized by hot air. The balanced equation will be calculated along with the. Iron(iii) oxide is also. Iron Iii Oxide And Hydrochloric Acid.
From www.showme.com
Iron III oxide Science, Chemistry, Chemical Bonds ShowMe Iron Iii Oxide And Hydrochloric Acid Iron(iii) oxide is also insoluble in water and. Chemical properties of iron(iii) oxide reaction with acids. 6hcl (aq) + fe2o3 (s) —+3h20 (1) + 2fecl3 (aq) we can interpret this to mean: This reaction demonstrates its basic nature. Iron(ii) oxide is a black solid that is insoluble in water and is easily oxidized by hot air. The balanced equation will. Iron Iii Oxide And Hydrochloric Acid.
From www.numerade.com
SOLVED 2 Write the equilibrium equation between elemental hydrogen and Iron Iii Oxide And Hydrochloric Acid 6hcl (aq) + fe2o3 (s) —+3h20 (1) + 2fecl3 (aq) we can interpret this to mean: Iron(iii) oxide reacts with acids, such as hydrochloric acid, to form iron(iii) chloride and water. Fe2o3 + hcl = fecl3 + h2o is a double displacement (metathesis) reaction where one mole of hematite [fe 2 o 3] and six moles of. Iron(ii) oxide is. Iron Iii Oxide And Hydrochloric Acid.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW Iron Iii Oxide And Hydrochloric Acid Now that we have discussed the various highlights of iron(iii) oxide we. Fe₂o₃ + 6𝐻𝐶𝑙 → 2𝐹𝑒𝐶𝑙₃ + 3𝐻₂𝑂; Iron(ii) oxide is a black solid that is insoluble in water and is easily oxidized by hot air. 6hcl (aq) + fe2o3 (s) —+3h20 (1) + 2fecl3 (aq) we can interpret this to mean: The iron(iii) oxide and sulfuric acid react. Iron Iii Oxide And Hydrochloric Acid.
From www.numerade.com
SOLVED 1 Iron III) oxide + hydrochloric acid gives you Iron (II Iron Iii Oxide And Hydrochloric Acid Iron(iii) oxide reacts with acids, such as hydrochloric acid, to form iron(iii) chloride and water. This reaction demonstrates its basic nature. The balanced equation will be calculated along with the. Chemical properties of iron(iii) oxide reaction with acids. Iron(ii) oxide is a black solid that is insoluble in water and is easily oxidized by hot air. The iron(iii) oxide and. Iron Iii Oxide And Hydrochloric Acid.
From www.numerade.com
SOLVED [References] Use the References to access important values if Iron Iii Oxide And Hydrochloric Acid Chemical properties of iron(iii) oxide reaction with acids. Fe2o3 + hcl = fecl3 + h2o is a double displacement (metathesis) reaction where one mole of hematite [fe 2 o 3] and six moles of. Iron(iii) oxide is soluble in strong acids such as hydrochloric acid (hcl) and sulfuric acid (h 2 so 4). The balanced equation will be calculated along. Iron Iii Oxide And Hydrochloric Acid.
From www.wou.edu
CH150 Chapter 5 Chemical Reactions Chemistry Iron Iii Oxide And Hydrochloric Acid Iron(iii) oxide reacts with acids, such as hydrochloric acid, to form iron(iii) chloride and water. Iron(ii) oxide is a black solid that is insoluble in water and is easily oxidized by hot air. Iron(iii) oxide is soluble in strong acids such as hydrochloric acid (hcl) and sulfuric acid (h 2 so 4). The balanced equation will be calculated along with. Iron Iii Oxide And Hydrochloric Acid.
From www.sciencephoto.com
Iron (III) oxide in water and in hydrochloric acid Stock Image C036 Iron Iii Oxide And Hydrochloric Acid Iron(iii) oxide reacts with acids, such as hydrochloric acid, to form iron(iii) chloride and water. Chemical properties of iron(iii) oxide reaction with acids. Enter an equation of an ionic chemical equation and press the balance button. Iron(iii) oxide is soluble in strong acids such as hydrochloric acid (hcl) and sulfuric acid (h 2 so 4). The balanced equation will be. Iron Iii Oxide And Hydrochloric Acid.
From testbook.com
Iron(III) Oxide Formula Know Its Preparation, Properties & Uses Iron Iii Oxide And Hydrochloric Acid The balanced chemical equation for the reaction between hydrochloric acid and iron (iii) oxide is: Fe₂o₃ + 6𝐻𝐶𝑙 → 2𝐹𝑒𝐶𝑙₃ + 3𝐻₂𝑂; Iron(iii) oxide reacts with acids, such as hydrochloric acid, to form iron(iii) chloride and water. The balanced equation will be calculated along with the. 6hcl (aq) + fe2o3 (s) —+3h20 (1) + 2fecl3 (aq) we can interpret this. Iron Iii Oxide And Hydrochloric Acid.
From www.numerade.com
SOLVED 1. Write an equation showing the oxidation (rusting) of iron to Iron Iii Oxide And Hydrochloric Acid Fe₂o₃ + 6𝐻𝐶𝑙 → 2𝐹𝑒𝐶𝑙₃ + 3𝐻₂𝑂; Now that we have discussed the various highlights of iron(iii) oxide we. 6hcl (aq) + fe2o3 (s) —+3h20 (1) + 2fecl3 (aq) we can interpret this to mean: Iron(iii) oxide reacts with acids, such as hydrochloric acid, to form iron(iii) chloride and water. Enter an equation of an ionic chemical equation and press. Iron Iii Oxide And Hydrochloric Acid.
From www.coursehero.com
[Solved] The reaction of iron(III)sulphide (Fe 2 S 3 ) and hydrochloric Iron Iii Oxide And Hydrochloric Acid Iron(iii) oxide reacts with acids, such as hydrochloric acid, to form iron(iii) chloride and water. Fe₂o₃ + 6𝐻𝐶𝑙 → 2𝐹𝑒𝐶𝑙₃ + 3𝐻₂𝑂; The iron(iii) oxide and sulfuric acid react together under hot conditions and produce the salt iron(iii) sulfate as well. 6hcl (aq) + fe2o3 (s) —+3h20 (1) + 2fecl3 (aq) we can interpret this to mean: Enter an equation. Iron Iii Oxide And Hydrochloric Acid.
From www.numerade.com
SOLVED (e) aqueous barium hydroxide and sulfuric acid aqueous barium Iron Iii Oxide And Hydrochloric Acid Fe2o3 + hcl = fecl3 + h2o is a double displacement (metathesis) reaction where one mole of hematite [fe 2 o 3] and six moles of. Fe₂o₃ + 6𝐻𝐶𝑙 → 2𝐹𝑒𝐶𝑙₃ + 3𝐻₂𝑂; Iron(iii) oxide is soluble in strong acids such as hydrochloric acid (hcl) and sulfuric acid (h 2 so 4). Chemical properties of iron(iii) oxide reaction with acids.. Iron Iii Oxide And Hydrochloric Acid.
From www.numerade.com
SOLVED The chemical equation below represents the reaction between Iron Iii Oxide And Hydrochloric Acid This reaction demonstrates its basic nature. Now that we have discussed the various highlights of iron(iii) oxide we. The balanced equation will be calculated along with the. Iron(iii) oxide reacts with acids, such as hydrochloric acid, to form iron(iii) chloride and water. Iron(iii) oxide is soluble in strong acids such as hydrochloric acid (hcl) and sulfuric acid (h 2 so. Iron Iii Oxide And Hydrochloric Acid.
From www.chegg.com
Solved D. Iron combined with hydrochloric acid Iron Iii Oxide And Hydrochloric Acid Chemical properties of iron(iii) oxide reaction with acids. Now that we have discussed the various highlights of iron(iii) oxide we. The iron(iii) oxide and sulfuric acid react together under hot conditions and produce the salt iron(iii) sulfate as well. Fe₂o₃ + 6𝐻𝐶𝑙 → 2𝐹𝑒𝐶𝑙₃ + 3𝐻₂𝑂; This reaction demonstrates its basic nature. Fe2o3 + hcl = fecl3 + h2o is. Iron Iii Oxide And Hydrochloric Acid.
From www.chegg.com
Solved 11. Iron reacts with water to form iron (III) oxide Iron Iii Oxide And Hydrochloric Acid This reaction demonstrates its basic nature. Iron(ii) oxide is a black solid that is insoluble in water and is easily oxidized by hot air. Enter an equation of an ionic chemical equation and press the balance button. The balanced chemical equation for the reaction between hydrochloric acid and iron (iii) oxide is: Fe2o3 + hcl = fecl3 + h2o is. Iron Iii Oxide And Hydrochloric Acid.
From slideplayer.com
Metals & nonmetals a comparison of properties. ppt download Iron Iii Oxide And Hydrochloric Acid Iron(iii) oxide is soluble in strong acids such as hydrochloric acid (hcl) and sulfuric acid (h 2 so 4). Enter an equation of an ionic chemical equation and press the balance button. The balanced equation will be calculated along with the. Iron(iii) oxide reacts with acids, such as hydrochloric acid, to form iron(iii) chloride and water. 6hcl (aq) + fe2o3. Iron Iii Oxide And Hydrochloric Acid.
From www.researchgate.net
Mineral pigments in the iron(III) oxide/hydroxide system. Download Iron Iii Oxide And Hydrochloric Acid Iron(iii) oxide reacts with acids, such as hydrochloric acid, to form iron(iii) chloride and water. Fe2o3 + hcl = fecl3 + h2o is a double displacement (metathesis) reaction where one mole of hematite [fe 2 o 3] and six moles of. Now that we have discussed the various highlights of iron(iii) oxide we. Iron(iii) oxide is also insoluble in water. Iron Iii Oxide And Hydrochloric Acid.
From www.nagwa.com
Question Video Identifying the Products When a Metal Oxide Reacts with Iron Iii Oxide And Hydrochloric Acid The iron(iii) oxide and sulfuric acid react together under hot conditions and produce the salt iron(iii) sulfate as well. Iron(iii) oxide reacts with acids, such as hydrochloric acid, to form iron(iii) chloride and water. Now that we have discussed the various highlights of iron(iii) oxide we. Enter an equation of an ionic chemical equation and press the balance button. Chemical. Iron Iii Oxide And Hydrochloric Acid.
From www.youtube.com
35 Fe2O3 + HCl Iron(III) oxide + Hydrochloric acid💚 YouTube Iron Iii Oxide And Hydrochloric Acid Now that we have discussed the various highlights of iron(iii) oxide we. Iron(iii) oxide reacts with acids, such as hydrochloric acid, to form iron(iii) chloride and water. Iron(iii) oxide is soluble in strong acids such as hydrochloric acid (hcl) and sulfuric acid (h 2 so 4). The balanced chemical equation for the reaction between hydrochloric acid and iron (iii) oxide. Iron Iii Oxide And Hydrochloric Acid.
From hydrogengasgaosube.blogspot.com
Hydrogen Gas Bubbles Of Hydrogen Gas And Aqueous Iron (iii) Iron Iii Oxide And Hydrochloric Acid The balanced equation will be calculated along with the. Chemical properties of iron(iii) oxide reaction with acids. The balanced chemical equation for the reaction between hydrochloric acid and iron (iii) oxide is: This reaction demonstrates its basic nature. Enter an equation of an ionic chemical equation and press the balance button. The iron(iii) oxide and sulfuric acid react together under. Iron Iii Oxide And Hydrochloric Acid.
From www.slideserve.com
PPT Chapter 11 Chemical Reactions PowerPoint Presentation ID6634764 Iron Iii Oxide And Hydrochloric Acid Iron(ii) oxide is a black solid that is insoluble in water and is easily oxidized by hot air. The iron(iii) oxide and sulfuric acid react together under hot conditions and produce the salt iron(iii) sulfate as well. Iron(iii) oxide is also insoluble in water and. Now that we have discussed the various highlights of iron(iii) oxide we. The balanced equation. Iron Iii Oxide And Hydrochloric Acid.
From www.sciencephoto.com
Iron (III) oxide in hydrochloric acid Stock Image C036/3132 Iron Iii Oxide And Hydrochloric Acid Now that we have discussed the various highlights of iron(iii) oxide we. 6hcl (aq) + fe2o3 (s) —+3h20 (1) + 2fecl3 (aq) we can interpret this to mean: Iron(ii) oxide is a black solid that is insoluble in water and is easily oxidized by hot air. The iron(iii) oxide and sulfuric acid react together under hot conditions and produce the. Iron Iii Oxide And Hydrochloric Acid.
From www.numerade.com
SOLVED aqueous barium hydroxide and sulfuric acid aqueous barium Iron Iii Oxide And Hydrochloric Acid 6hcl (aq) + fe2o3 (s) —+3h20 (1) + 2fecl3 (aq) we can interpret this to mean: The balanced chemical equation for the reaction between hydrochloric acid and iron (iii) oxide is: Iron(iii) oxide reacts with acids, such as hydrochloric acid, to form iron(iii) chloride and water. Iron(iii) oxide is also insoluble in water and. Enter an equation of an ionic. Iron Iii Oxide And Hydrochloric Acid.
From www.hanlin.com
CIE A Level Chemistry复习笔记2.2.2 Reactions of Group 2 Oxides, Hydroxides Iron Iii Oxide And Hydrochloric Acid Iron(iii) oxide is also insoluble in water and. Fe₂o₃ + 6𝐻𝐶𝑙 → 2𝐹𝑒𝐶𝑙₃ + 3𝐻₂𝑂; Chemical properties of iron(iii) oxide reaction with acids. The balanced chemical equation for the reaction between hydrochloric acid and iron (iii) oxide is: The balanced equation will be calculated along with the. Iron(iii) oxide is soluble in strong acids such as hydrochloric acid (hcl) and. Iron Iii Oxide And Hydrochloric Acid.
From www.chegg.com
Solved When iron(III) oxide reacts with hydrochloric acid, Iron Iii Oxide And Hydrochloric Acid The balanced chemical equation for the reaction between hydrochloric acid and iron (iii) oxide is: 6hcl (aq) + fe2o3 (s) —+3h20 (1) + 2fecl3 (aq) we can interpret this to mean: Fe2o3 + hcl = fecl3 + h2o is a double displacement (metathesis) reaction where one mole of hematite [fe 2 o 3] and six moles of. This reaction demonstrates. Iron Iii Oxide And Hydrochloric Acid.
From brainly.com
Iron (III) Oxide reacting with Hydrochloric Acid to produce Iron Iron Iii Oxide And Hydrochloric Acid The balanced equation will be calculated along with the. Now that we have discussed the various highlights of iron(iii) oxide we. Fe₂o₃ + 6𝐻𝐶𝑙 → 2𝐹𝑒𝐶𝑙₃ + 3𝐻₂𝑂; Enter an equation of an ionic chemical equation and press the balance button. Iron(iii) oxide is also insoluble in water and. Fe2o3 + hcl = fecl3 + h2o is a double displacement. Iron Iii Oxide And Hydrochloric Acid.
From www.numerade.com
SOLVED For the following reaction, 4.09 grams of hydrochloric acid are Iron Iii Oxide And Hydrochloric Acid Fe2o3 + hcl = fecl3 + h2o is a double displacement (metathesis) reaction where one mole of hematite [fe 2 o 3] and six moles of. Iron(iii) oxide is also insoluble in water and. Iron(iii) oxide is soluble in strong acids such as hydrochloric acid (hcl) and sulfuric acid (h 2 so 4). The balanced equation will be calculated along. Iron Iii Oxide And Hydrochloric Acid.
From www.youtube.com
How to Write the Formula for Iron (III) Oxide YouTube Iron Iii Oxide And Hydrochloric Acid This reaction demonstrates its basic nature. The iron(iii) oxide and sulfuric acid react together under hot conditions and produce the salt iron(iii) sulfate as well. 6hcl (aq) + fe2o3 (s) —+3h20 (1) + 2fecl3 (aq) we can interpret this to mean: Iron(iii) oxide is also insoluble in water and. The balanced equation will be calculated along with the. Enter an. Iron Iii Oxide And Hydrochloric Acid.
From www.youtube.com
Redox Reaction Oxidation of Iron (II) to Iron (III) YouTube Iron Iii Oxide And Hydrochloric Acid Iron(iii) oxide is also insoluble in water and. This reaction demonstrates its basic nature. Fe2o3 + hcl = fecl3 + h2o is a double displacement (metathesis) reaction where one mole of hematite [fe 2 o 3] and six moles of. The iron(iii) oxide and sulfuric acid react together under hot conditions and produce the salt iron(iii) sulfate as well. Fe₂o₃. Iron Iii Oxide And Hydrochloric Acid.
From studylib.net
Extraction of Iron III with Tributylphosphine Oxide from Hydrochloric Iron Iii Oxide And Hydrochloric Acid Fe2o3 + hcl = fecl3 + h2o is a double displacement (metathesis) reaction where one mole of hematite [fe 2 o 3] and six moles of. The balanced equation will be calculated along with the. 6hcl (aq) + fe2o3 (s) —+3h20 (1) + 2fecl3 (aq) we can interpret this to mean: Iron(iii) oxide is also insoluble in water and. Chemical. Iron Iii Oxide And Hydrochloric Acid.
From www.youtube.com
Balance Fe + HCl = FeCl2 + H2 (Iron and Hydrochloric Acid) YouTube Iron Iii Oxide And Hydrochloric Acid Enter an equation of an ionic chemical equation and press the balance button. 6hcl (aq) + fe2o3 (s) —+3h20 (1) + 2fecl3 (aq) we can interpret this to mean: Now that we have discussed the various highlights of iron(iii) oxide we. The balanced equation will be calculated along with the. Iron(ii) oxide is a black solid that is insoluble in. Iron Iii Oxide And Hydrochloric Acid.
From www.showme.com
Write the formula for iron (III) oxide and iron (III) sulfate Iron Iii Oxide And Hydrochloric Acid Iron(iii) oxide is soluble in strong acids such as hydrochloric acid (hcl) and sulfuric acid (h 2 so 4). The iron(iii) oxide and sulfuric acid react together under hot conditions and produce the salt iron(iii) sulfate as well. Iron(iii) oxide reacts with acids, such as hydrochloric acid, to form iron(iii) chloride and water. Now that we have discussed the various. Iron Iii Oxide And Hydrochloric Acid.
From www.numerade.com
SOLVED For the following reaction, 6.84 grams of hydrochloric acid are Iron Iii Oxide And Hydrochloric Acid Iron(ii) oxide is a black solid that is insoluble in water and is easily oxidized by hot air. This reaction demonstrates its basic nature. The iron(iii) oxide and sulfuric acid react together under hot conditions and produce the salt iron(iii) sulfate as well. Chemical properties of iron(iii) oxide reaction with acids. Iron(iii) oxide reacts with acids, such as hydrochloric acid,. Iron Iii Oxide And Hydrochloric Acid.