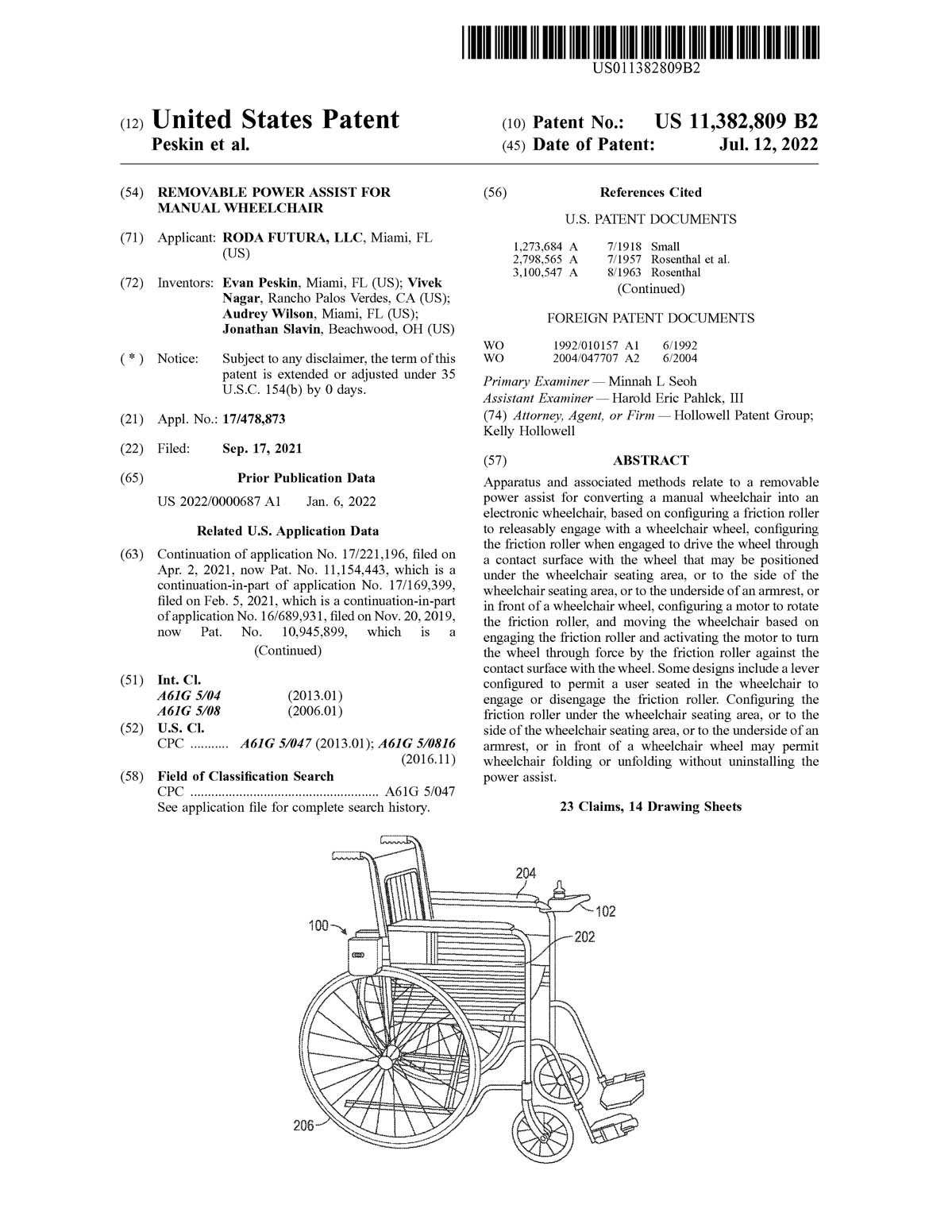
Medical Device Patent Term Extension . 156 (f) (2)), and to medical devices, food. Patent term restoration is available for certain patents related to drug products (as defined in 35 u.s.c. A patent term extension under 35 u.s.c. Patent term extension is provided in canada in the form of a certificate of supplementary protection (csp). A csp is available only for the. General requirements for, and contents of, patent term extension (pte) application. 156 is a limited extension of the patent rights associated with the approved product. § 156 may be filed for patents on certain human drugs, food or color. Medical devices are subject to review under section 156(f)(1)(b). Thus, patents covering medical devices may be eligible for. The statute enables the owners of patents on human drug products, medical devices, food additives, or color additives, and animal. Applications for patent term extension under 35 u.s.c. The act allows the extension of the term of a patent claiming a product that requires regulatory approval prior to being sold, or a method of.
from hpgww.com
A csp is available only for the. General requirements for, and contents of, patent term extension (pte) application. 156 is a limited extension of the patent rights associated with the approved product. Medical devices are subject to review under section 156(f)(1)(b). § 156 may be filed for patents on certain human drugs, food or color. Thus, patents covering medical devices may be eligible for. A patent term extension under 35 u.s.c. The act allows the extension of the term of a patent claiming a product that requires regulatory approval prior to being sold, or a method of. 156 (f) (2)), and to medical devices, food. Patent term extension is provided in canada in the form of a certificate of supplementary protection (csp).
Medical Device Patent Attorneys Hollowell Patent Group
Medical Device Patent Term Extension Medical devices are subject to review under section 156(f)(1)(b). Applications for patent term extension under 35 u.s.c. 156 (f) (2)), and to medical devices, food. Medical devices are subject to review under section 156(f)(1)(b). A csp is available only for the. A patent term extension under 35 u.s.c. General requirements for, and contents of, patent term extension (pte) application. The statute enables the owners of patents on human drug products, medical devices, food additives, or color additives, and animal. Thus, patents covering medical devices may be eligible for. The act allows the extension of the term of a patent claiming a product that requires regulatory approval prior to being sold, or a method of. Patent term extension is provided in canada in the form of a certificate of supplementary protection (csp). Patent term restoration is available for certain patents related to drug products (as defined in 35 u.s.c. § 156 may be filed for patents on certain human drugs, food or color. 156 is a limited extension of the patent rights associated with the approved product.
From patentbusinesslawyer.com
Patent Case Study Medical Device Patents Medical Device Patent Term Extension Applications for patent term extension under 35 u.s.c. General requirements for, and contents of, patent term extension (pte) application. The act allows the extension of the term of a patent claiming a product that requires regulatory approval prior to being sold, or a method of. Thus, patents covering medical devices may be eligible for. A csp is available only for. Medical Device Patent Term Extension.
From www.scribd.com
Notice Medical Devices Patent Extension Regulatory Review Period Medical Device Patent Term Extension A patent term extension under 35 u.s.c. Patent term restoration is available for certain patents related to drug products (as defined in 35 u.s.c. Medical devices are subject to review under section 156(f)(1)(b). 156 is a limited extension of the patent rights associated with the approved product. The statute enables the owners of patents on human drug products, medical devices,. Medical Device Patent Term Extension.
From www.slideshare.net
Patent Exhaustion of Medical Device Patents Lifescan v. Shasta by Medical Device Patent Term Extension Thus, patents covering medical devices may be eligible for. Applications for patent term extension under 35 u.s.c. A csp is available only for the. Medical devices are subject to review under section 156(f)(1)(b). § 156 may be filed for patents on certain human drugs, food or color. Patent term extension is provided in canada in the form of a certificate. Medical Device Patent Term Extension.
From data.epo.org
MEDICAL DEVICE Patent 4205791 Medical Device Patent Term Extension Patent term extension is provided in canada in the form of a certificate of supplementary protection (csp). 156 is a limited extension of the patent rights associated with the approved product. The statute enables the owners of patents on human drug products, medical devices, food additives, or color additives, and animal. Medical devices are subject to review under section 156(f)(1)(b).. Medical Device Patent Term Extension.
From remmed.com
How to File for a Medical Device Patent Remington Medical Device Patent Term Extension Patent term extension is provided in canada in the form of a certificate of supplementary protection (csp). The statute enables the owners of patents on human drug products, medical devices, food additives, or color additives, and animal. § 156 may be filed for patents on certain human drugs, food or color. 156 (f) (2)), and to medical devices, food. A. Medical Device Patent Term Extension.
From arapackelaw.com
The MustHave Medical Device Patent Guide The Rapacke Law Group Medical Device Patent Term Extension Applications for patent term extension under 35 u.s.c. 156 (f) (2)), and to medical devices, food. The statute enables the owners of patents on human drug products, medical devices, food additives, or color additives, and animal. § 156 may be filed for patents on certain human drugs, food or color. 156 is a limited extension of the patent rights associated. Medical Device Patent Term Extension.
From www.drugpatentwatch.com
Understanding Patent Term Extensions An Overview DrugPatentWatch Medical Device Patent Term Extension Patent term extension is provided in canada in the form of a certificate of supplementary protection (csp). Thus, patents covering medical devices may be eligible for. A csp is available only for the. General requirements for, and contents of, patent term extension (pte) application. The act allows the extension of the term of a patent claiming a product that requires. Medical Device Patent Term Extension.
From www.slideshare.net
Medical devices patent trends technical, legal & regulatory consultan… Medical Device Patent Term Extension The statute enables the owners of patents on human drug products, medical devices, food additives, or color additives, and animal. Patent term extension is provided in canada in the form of a certificate of supplementary protection (csp). § 156 may be filed for patents on certain human drugs, food or color. 156 is a limited extension of the patent rights. Medical Device Patent Term Extension.
From arapackelaw.com
Patent Term Extension Learn How Your Medical Device Inventions Can Medical Device Patent Term Extension The act allows the extension of the term of a patent claiming a product that requires regulatory approval prior to being sold, or a method of. The statute enables the owners of patents on human drug products, medical devices, food additives, or color additives, and animal. A patent term extension under 35 u.s.c. 156 (f) (2)), and to medical devices,. Medical Device Patent Term Extension.
From www.scribd.com
Notice Medical Devices Patent Extension Regulatory Review Period Medical Device Patent Term Extension The statute enables the owners of patents on human drug products, medical devices, food additives, or color additives, and animal. § 156 may be filed for patents on certain human drugs, food or color. A patent term extension under 35 u.s.c. General requirements for, and contents of, patent term extension (pte) application. 156 (f) (2)), and to medical devices, food.. Medical Device Patent Term Extension.
From www.scribd.com
Notice Medical Devices Patent Extension Regulatory Review Period Medical Device Patent Term Extension 156 (f) (2)), and to medical devices, food. Patent term restoration is available for certain patents related to drug products (as defined in 35 u.s.c. The statute enables the owners of patents on human drug products, medical devices, food additives, or color additives, and animal. Medical devices are subject to review under section 156(f)(1)(b). The act allows the extension of. Medical Device Patent Term Extension.
From creanova.com
Medical device design patent What, When and Why? Creanova Medical Device Patent Term Extension Patent term restoration is available for certain patents related to drug products (as defined in 35 u.s.c. 156 (f) (2)), and to medical devices, food. 156 is a limited extension of the patent rights associated with the approved product. General requirements for, and contents of, patent term extension (pte) application. Patent term extension is provided in canada in the form. Medical Device Patent Term Extension.
From arapackelaw.com
Types of Medical Device Patents The Rapacke Law Group Medical Device Patent Term Extension A patent term extension under 35 u.s.c. 156 (f) (2)), and to medical devices, food. Thus, patents covering medical devices may be eligible for. The act allows the extension of the term of a patent claiming a product that requires regulatory approval prior to being sold, or a method of. Patent term restoration is available for certain patents related to. Medical Device Patent Term Extension.
From powerpatent.com
Analyzing Medical Devices Patents Latest Medical Device Patent Medical Device Patent Term Extension Patent term extension is provided in canada in the form of a certificate of supplementary protection (csp). 156 is a limited extension of the patent rights associated with the approved product. General requirements for, and contents of, patent term extension (pte) application. Medical devices are subject to review under section 156(f)(1)(b). A patent term extension under 35 u.s.c. The statute. Medical Device Patent Term Extension.
From www.levinconsultinggroup.com
How to Patent a Medical Device Levin Consulting Group Medical Device Patent Term Extension Patent term extension is provided in canada in the form of a certificate of supplementary protection (csp). Thus, patents covering medical devices may be eligible for. § 156 may be filed for patents on certain human drugs, food or color. General requirements for, and contents of, patent term extension (pte) application. Applications for patent term extension under 35 u.s.c. The. Medical Device Patent Term Extension.
From www.slideserve.com
PPT Patent Term Adjustments and Extensions PowerPoint Presentation Medical Device Patent Term Extension A csp is available only for the. Applications for patent term extension under 35 u.s.c. The statute enables the owners of patents on human drug products, medical devices, food additives, or color additives, and animal. Thus, patents covering medical devices may be eligible for. Patent term restoration is available for certain patents related to drug products (as defined in 35. Medical Device Patent Term Extension.
From www.scribd.com
Notice Medical Devices Patent Extension Regulatory Review Period Medical Device Patent Term Extension Thus, patents covering medical devices may be eligible for. A patent term extension under 35 u.s.c. § 156 may be filed for patents on certain human drugs, food or color. The statute enables the owners of patents on human drug products, medical devices, food additives, or color additives, and animal. Medical devices are subject to review under section 156(f)(1)(b). Patent. Medical Device Patent Term Extension.
From arapackelaw.com
Patent Term Extension Learn How Your Medical Device Inventions Can Medical Device Patent Term Extension Patent term restoration is available for certain patents related to drug products (as defined in 35 u.s.c. A csp is available only for the. Patent term extension is provided in canada in the form of a certificate of supplementary protection (csp). 156 is a limited extension of the patent rights associated with the approved product. Applications for patent term extension. Medical Device Patent Term Extension.
From www.biosimilarsip.com
Brazilian Supreme Court Ends Patent Term Extension and Retroactively Medical Device Patent Term Extension Patent term restoration is available for certain patents related to drug products (as defined in 35 u.s.c. § 156 may be filed for patents on certain human drugs, food or color. Applications for patent term extension under 35 u.s.c. Patent term extension is provided in canada in the form of a certificate of supplementary protection (csp). A csp is available. Medical Device Patent Term Extension.
From arapackelaw.com
The MustHave Medical Device Patent Guide Resources Library Medical Device Patent Term Extension A csp is available only for the. Medical devices are subject to review under section 156(f)(1)(b). Thus, patents covering medical devices may be eligible for. General requirements for, and contents of, patent term extension (pte) application. 156 (f) (2)), and to medical devices, food. A patent term extension under 35 u.s.c. § 156 may be filed for patents on certain. Medical Device Patent Term Extension.
From pressbooks.umn.edu
Patent Basics Medical Device Innovation Handbook Medical Device Patent Term Extension Patent term extension is provided in canada in the form of a certificate of supplementary protection (csp). A csp is available only for the. General requirements for, and contents of, patent term extension (pte) application. 156 (f) (2)), and to medical devices, food. Medical devices are subject to review under section 156(f)(1)(b). 156 is a limited extension of the patent. Medical Device Patent Term Extension.
From www.slideserve.com
PPT Overview Of Medical Device Patent Process PowerPoint Presentation Medical Device Patent Term Extension Medical devices are subject to review under section 156(f)(1)(b). The statute enables the owners of patents on human drug products, medical devices, food additives, or color additives, and animal. A patent term extension under 35 u.s.c. The act allows the extension of the term of a patent claiming a product that requires regulatory approval prior to being sold, or a. Medical Device Patent Term Extension.
From www.iancollmceachern.com
Understanding the Different Types of Medical Device Patents Medical Device Patent Term Extension A csp is available only for the. A patent term extension under 35 u.s.c. General requirements for, and contents of, patent term extension (pte) application. Medical devices are subject to review under section 156(f)(1)(b). Patent term extension is provided in canada in the form of a certificate of supplementary protection (csp). Patent term restoration is available for certain patents related. Medical Device Patent Term Extension.
From www.goldsteinpatentlaw.com
How To Patent Medical Devices In 3 Steps Goldstein Patent Law Medical Device Patent Term Extension Medical devices are subject to review under section 156(f)(1)(b). Patent term restoration is available for certain patents related to drug products (as defined in 35 u.s.c. Applications for patent term extension under 35 u.s.c. Thus, patents covering medical devices may be eligible for. A patent term extension under 35 u.s.c. 156 (f) (2)), and to medical devices, food. 156 is. Medical Device Patent Term Extension.
From www.slideserve.com
PPT Japan ’ s Healthcare System and Generic Drug Industry PowerPoint Medical Device Patent Term Extension § 156 may be filed for patents on certain human drugs, food or color. A patent term extension under 35 u.s.c. Patent term restoration is available for certain patents related to drug products (as defined in 35 u.s.c. A csp is available only for the. 156 is a limited extension of the patent rights associated with the approved product. Patent. Medical Device Patent Term Extension.
From hpgww.com
Medical Device Patent Attorneys Hollowell Patent Group Medical Device Patent Term Extension General requirements for, and contents of, patent term extension (pte) application. A csp is available only for the. Patent term restoration is available for certain patents related to drug products (as defined in 35 u.s.c. Applications for patent term extension under 35 u.s.c. The act allows the extension of the term of a patent claiming a product that requires regulatory. Medical Device Patent Term Extension.
From hpgww.com
Medical Device Patent Attorneys Hollowell Patent Group Medical Device Patent Term Extension Medical devices are subject to review under section 156(f)(1)(b). § 156 may be filed for patents on certain human drugs, food or color. The act allows the extension of the term of a patent claiming a product that requires regulatory approval prior to being sold, or a method of. 156 (f) (2)), and to medical devices, food. Applications for patent. Medical Device Patent Term Extension.
From www.intepat.com
What is Patent Term Extension India Position Intepat Medical Device Patent Term Extension The act allows the extension of the term of a patent claiming a product that requires regulatory approval prior to being sold, or a method of. A patent term extension under 35 u.s.c. General requirements for, and contents of, patent term extension (pte) application. 156 is a limited extension of the patent rights associated with the approved product. Patent term. Medical Device Patent Term Extension.
From innovations.bmj.com
Indian medical device sector Insights from patent filing trends BMJ Medical Device Patent Term Extension Medical devices are subject to review under section 156(f)(1)(b). Applications for patent term extension under 35 u.s.c. A csp is available only for the. 156 is a limited extension of the patent rights associated with the approved product. The act allows the extension of the term of a patent claiming a product that requires regulatory approval prior to being sold,. Medical Device Patent Term Extension.
From www.gilero.com
Understanding Medical Device Patents Gilero Medical Device Patent Term Extension 156 is a limited extension of the patent rights associated with the approved product. Applications for patent term extension under 35 u.s.c. Patent term extension is provided in canada in the form of a certificate of supplementary protection (csp). Patent term restoration is available for certain patents related to drug products (as defined in 35 u.s.c. A csp is available. Medical Device Patent Term Extension.
From patentlawyer.io
Understanding the Importance of Patents in the Medical Device Industry Medical Device Patent Term Extension A patent term extension under 35 u.s.c. Medical devices are subject to review under section 156(f)(1)(b). The statute enables the owners of patents on human drug products, medical devices, food additives, or color additives, and animal. Thus, patents covering medical devices may be eligible for. § 156 may be filed for patents on certain human drugs, food or color. 156. Medical Device Patent Term Extension.
From www.slideserve.com
PPT Patent Term Adjustments and Extensions PowerPoint Presentation Medical Device Patent Term Extension The statute enables the owners of patents on human drug products, medical devices, food additives, or color additives, and animal. The act allows the extension of the term of a patent claiming a product that requires regulatory approval prior to being sold, or a method of. Patent term extension is provided in canada in the form of a certificate of. Medical Device Patent Term Extension.
From patentbusinesslawyer.com
Medical Device Patent Case Study Medical Device Patent Term Extension 156 is a limited extension of the patent rights associated with the approved product. 156 (f) (2)), and to medical devices, food. The statute enables the owners of patents on human drug products, medical devices, food additives, or color additives, and animal. A patent term extension under 35 u.s.c. The act allows the extension of the term of a patent. Medical Device Patent Term Extension.
From www.biosimilarsip.com
Brazilian Supreme Court Ends Patent Term Extension and Retroactively Medical Device Patent Term Extension § 156 may be filed for patents on certain human drugs, food or color. A csp is available only for the. The statute enables the owners of patents on human drug products, medical devices, food additives, or color additives, and animal. General requirements for, and contents of, patent term extension (pte) application. A patent term extension under 35 u.s.c. The. Medical Device Patent Term Extension.
From www.biosimilarsip.com
Brazilian Supreme Court Ends Patent Term Extension and Retroactively Medical Device Patent Term Extension Medical devices are subject to review under section 156(f)(1)(b). Patent term restoration is available for certain patents related to drug products (as defined in 35 u.s.c. § 156 may be filed for patents on certain human drugs, food or color. A patent term extension under 35 u.s.c. A csp is available only for the. Thus, patents covering medical devices may. Medical Device Patent Term Extension.