Threshold Energy And Activation Energy Difference . the difference between threshold energy and average energy of reactant molecules is called: Threshold energy = energy of normal molecules +. in chemical reactions, the energy barrier corresponds to the amount of energy the particles must have to react when they collide. activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the. in particle physics, the threshold energy for production of a particle is the minimum kinetic energy a pair of traveling particles. The difference between these two values. in the arrhenius model of reaction rates, activation energy is the minimum amount of energy that must be available to reactants. the activation energy (ea), labeled δg ‡ in figure 2, is the energy difference between the reactants and the activated complex, also known as transition state. — activation energy plays a crucial role in determining the rate of a chemical reaction; — the threshold energy is the energy that these molecules must have in order for a reaction to take place.
from circuitwiringtray.z13.web.core.windows.net
— the threshold energy is the energy that these molecules must have in order for a reaction to take place. activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the. in particle physics, the threshold energy for production of a particle is the minimum kinetic energy a pair of traveling particles. in the arrhenius model of reaction rates, activation energy is the minimum amount of energy that must be available to reactants. the activation energy (ea), labeled δg ‡ in figure 2, is the energy difference between the reactants and the activated complex, also known as transition state. — activation energy plays a crucial role in determining the rate of a chemical reaction; in chemical reactions, the energy barrier corresponds to the amount of energy the particles must have to react when they collide. The difference between these two values. Threshold energy = energy of normal molecules +. the difference between threshold energy and average energy of reactant molecules is called:
Diagram Of Activation Energy
Threshold Energy And Activation Energy Difference in chemical reactions, the energy barrier corresponds to the amount of energy the particles must have to react when they collide. — the threshold energy is the energy that these molecules must have in order for a reaction to take place. in the arrhenius model of reaction rates, activation energy is the minimum amount of energy that must be available to reactants. The difference between these two values. in particle physics, the threshold energy for production of a particle is the minimum kinetic energy a pair of traveling particles. the difference between threshold energy and average energy of reactant molecules is called: in chemical reactions, the energy barrier corresponds to the amount of energy the particles must have to react when they collide. Threshold energy = energy of normal molecules +. activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the. the activation energy (ea), labeled δg ‡ in figure 2, is the energy difference between the reactants and the activated complex, also known as transition state. — activation energy plays a crucial role in determining the rate of a chemical reaction;
From www.periodic-table.org
What is Critical Energy Threshold Energy for Fission Definition Threshold Energy And Activation Energy Difference in particle physics, the threshold energy for production of a particle is the minimum kinetic energy a pair of traveling particles. — activation energy plays a crucial role in determining the rate of a chemical reaction; — the threshold energy is the energy that these molecules must have in order for a reaction to take place. . Threshold Energy And Activation Energy Difference.
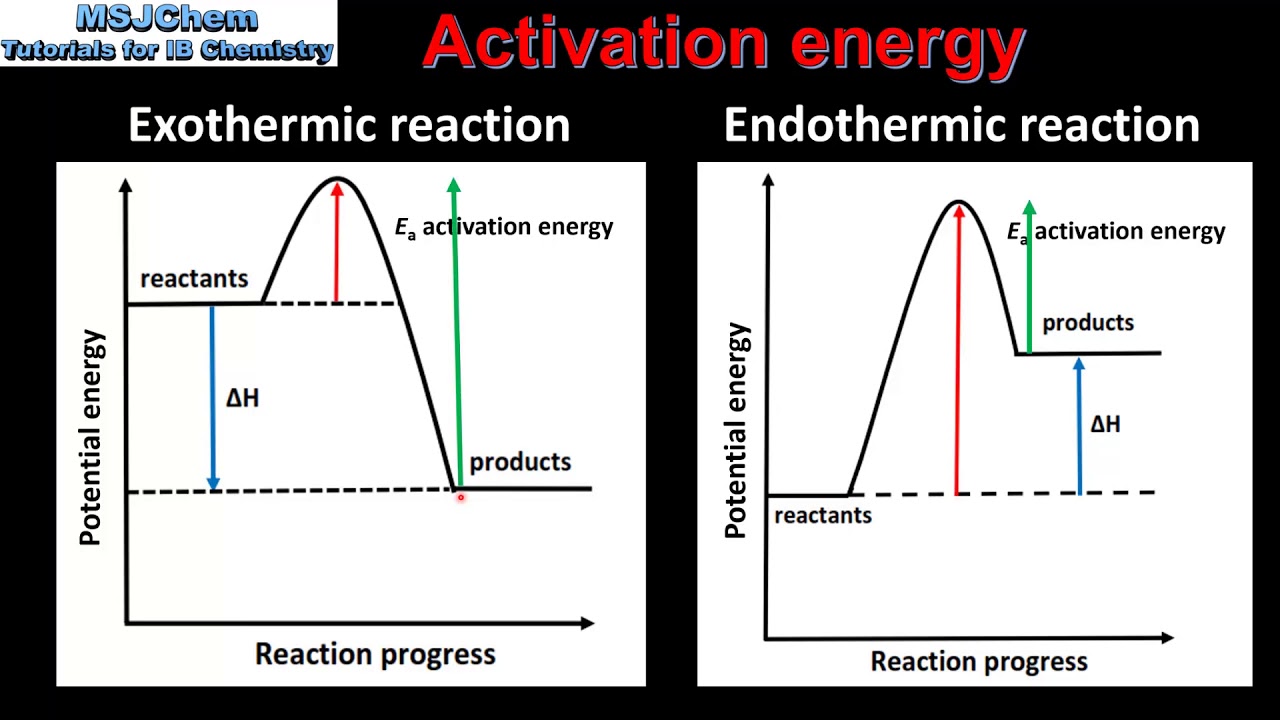
From www.youtube.com
Difference between Activation energy and Threshold energy YouTube Threshold Energy And Activation Energy Difference in the arrhenius model of reaction rates, activation energy is the minimum amount of energy that must be available to reactants. — activation energy plays a crucial role in determining the rate of a chemical reaction; activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the. in. Threshold Energy And Activation Energy Difference.
From nesslabs.com
Activation energy the chemistry of getting started Ness Labs Threshold Energy And Activation Energy Difference in particle physics, the threshold energy for production of a particle is the minimum kinetic energy a pair of traveling particles. — activation energy plays a crucial role in determining the rate of a chemical reaction; in the arrhenius model of reaction rates, activation energy is the minimum amount of energy that must be available to reactants.. Threshold Energy And Activation Energy Difference.
From www.differencebetween.com
Difference Between Energy and Activation Energy Compare the Threshold Energy And Activation Energy Difference The difference between these two values. — the threshold energy is the energy that these molecules must have in order for a reaction to take place. in chemical reactions, the energy barrier corresponds to the amount of energy the particles must have to react when they collide. activation energy represents the minimum energy required for a chemical. Threshold Energy And Activation Energy Difference.
From www.vrogue.co
What The Significance Of “er” In The Diagram A Av vrogue.co Threshold Energy And Activation Energy Difference in chemical reactions, the energy barrier corresponds to the amount of energy the particles must have to react when they collide. activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the. in particle physics, the threshold energy for production of a particle is the minimum kinetic energy a. Threshold Energy And Activation Energy Difference.
From circuitwiringtray.z13.web.core.windows.net
Diagram Of Activation Energy Threshold Energy And Activation Energy Difference activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the. in chemical reactions, the energy barrier corresponds to the amount of energy the particles must have to react when they collide. Threshold energy = energy of normal molecules +. the activation energy (ea), labeled δg ‡ in figure. Threshold Energy And Activation Energy Difference.
From www.youtube.com
Difference between Threshold energy and activation energy.Lecture 2 of Threshold Energy And Activation Energy Difference — the threshold energy is the energy that these molecules must have in order for a reaction to take place. activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the. in chemical reactions, the energy barrier corresponds to the amount of energy the particles must have to react. Threshold Energy And Activation Energy Difference.
From circuitwiringtray.z13.web.core.windows.net
Diagram Of Activation Energy Threshold Energy And Activation Energy Difference the activation energy (ea), labeled δg ‡ in figure 2, is the energy difference between the reactants and the activated complex, also known as transition state. The difference between these two values. — activation energy plays a crucial role in determining the rate of a chemical reaction; in the arrhenius model of reaction rates, activation energy is. Threshold Energy And Activation Energy Difference.
From classnotes.org.in
Arrhenius Equation and Activation Energy Chemical Chemistry Threshold Energy And Activation Energy Difference in chemical reactions, the energy barrier corresponds to the amount of energy the particles must have to react when they collide. activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the. the activation energy (ea), labeled δg ‡ in figure 2, is the energy difference between the reactants. Threshold Energy And Activation Energy Difference.
From www.doubtnut.com
[Odia] Define threshold energy and activation energy. How are they rel Threshold Energy And Activation Energy Difference the difference between threshold energy and average energy of reactant molecules is called: — the threshold energy is the energy that these molecules must have in order for a reaction to take place. in chemical reactions, the energy barrier corresponds to the amount of energy the particles must have to react when they collide. activation energy. Threshold Energy And Activation Energy Difference.
From www.numerade.com
SOLVEDThe activation energy for given reaction is (i.e., reactant → Threshold Energy And Activation Energy Difference in particle physics, the threshold energy for production of a particle is the minimum kinetic energy a pair of traveling particles. Threshold energy = energy of normal molecules +. activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the. in the arrhenius model of reaction rates, activation energy. Threshold Energy And Activation Energy Difference.
From www.chemistrylearner.com
Activation Energy Definition, Formula, and Graph Threshold Energy And Activation Energy Difference — activation energy plays a crucial role in determining the rate of a chemical reaction; activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the. in the arrhenius model of reaction rates, activation energy is the minimum amount of energy that must be available to reactants. the. Threshold Energy And Activation Energy Difference.
From circuitdbplastered.z13.web.core.windows.net
Reaction Energy Diagram With Catalyst Threshold Energy And Activation Energy Difference the difference between threshold energy and average energy of reactant molecules is called: activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the. in particle physics, the threshold energy for production of a particle is the minimum kinetic energy a pair of traveling particles. in chemical reactions,. Threshold Energy And Activation Energy Difference.
From byjus.com
What happens when the energy of a reaction if more than threshold Threshold Energy And Activation Energy Difference in particle physics, the threshold energy for production of a particle is the minimum kinetic energy a pair of traveling particles. activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the. the activation energy (ea), labeled δg ‡ in figure 2, is the energy difference between the reactants. Threshold Energy And Activation Energy Difference.
From ar.inspiredpencil.com
Activation Energy Example Threshold Energy And Activation Energy Difference — the threshold energy is the energy that these molecules must have in order for a reaction to take place. The difference between these two values. the activation energy (ea), labeled δg ‡ in figure 2, is the energy difference between the reactants and the activated complex, also known as transition state. in particle physics, the threshold. Threshold Energy And Activation Energy Difference.
From telegra.ph
Difference between activation energy and heat of reaction values Threshold Energy And Activation Energy Difference The difference between these two values. the activation energy (ea), labeled δg ‡ in figure 2, is the energy difference between the reactants and the activated complex, also known as transition state. — the threshold energy is the energy that these molecules must have in order for a reaction to take place. the difference between threshold energy. Threshold Energy And Activation Energy Difference.
From www.alamy.com
Graph of Progress of Reaction and Threshold energy Stock Vector Image Threshold Energy And Activation Energy Difference in particle physics, the threshold energy for production of a particle is the minimum kinetic energy a pair of traveling particles. The difference between these two values. — activation energy plays a crucial role in determining the rate of a chemical reaction; activation energy represents the minimum energy required for a chemical reaction to occur, while threshold. Threshold Energy And Activation Energy Difference.
From www.youtube.com
activation energy,threshold energy and Arrhenius equation in Hindi Threshold Energy And Activation Energy Difference activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the. the difference between threshold energy and average energy of reactant molecules is called: The difference between these two values. — the threshold energy is the energy that these molecules must have in order for a reaction to take. Threshold Energy And Activation Energy Difference.
From byjus.com
26. What is difference between threshold energy and activation energy? Threshold Energy And Activation Energy Difference — activation energy plays a crucial role in determining the rate of a chemical reaction; activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the. — the threshold energy is the energy that these molecules must have in order for a reaction to take place. in particle. Threshold Energy And Activation Energy Difference.
From www.differencebetween.com
Difference Between Activation Energy and Threshold Energy Compare the Threshold Energy And Activation Energy Difference in particle physics, the threshold energy for production of a particle is the minimum kinetic energy a pair of traveling particles. activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the. Threshold energy = energy of normal molecules +. in chemical reactions, the energy barrier corresponds to the. Threshold Energy And Activation Energy Difference.
From chemistry.stackexchange.com
physical chemistry How exactly is activation energy defined Threshold Energy And Activation Energy Difference — the threshold energy is the energy that these molecules must have in order for a reaction to take place. the activation energy (ea), labeled δg ‡ in figure 2, is the energy difference between the reactants and the activated complex, also known as transition state. activation energy represents the minimum energy required for a chemical reaction. Threshold Energy And Activation Energy Difference.
From www.sarthaks.com
The energy required to form the intermediate, called activated complex Threshold Energy And Activation Energy Difference activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the. — the threshold energy is the energy that these molecules must have in order for a reaction to take place. in particle physics, the threshold energy for production of a particle is the minimum kinetic energy a pair. Threshold Energy And Activation Energy Difference.
From douglasnewssantana.blogspot.com
Activation Energy Can Be Described as Threshold Energy And Activation Energy Difference in chemical reactions, the energy barrier corresponds to the amount of energy the particles must have to react when they collide. The difference between these two values. activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the. the difference between threshold energy and average energy of reactant molecules. Threshold Energy And Activation Energy Difference.
From www.askdifference.com
Energy vs. Activation Energy — What’s the Difference? Threshold Energy And Activation Energy Difference in the arrhenius model of reaction rates, activation energy is the minimum amount of energy that must be available to reactants. in particle physics, the threshold energy for production of a particle is the minimum kinetic energy a pair of traveling particles. the difference between threshold energy and average energy of reactant molecules is called: activation. Threshold Energy And Activation Energy Difference.
From ar.inspiredpencil.com
Activation Energy Diagram Endothermic Threshold Energy And Activation Energy Difference in chemical reactions, the energy barrier corresponds to the amount of energy the particles must have to react when they collide. — activation energy plays a crucial role in determining the rate of a chemical reaction; in the arrhenius model of reaction rates, activation energy is the minimum amount of energy that must be available to reactants.. Threshold Energy And Activation Energy Difference.
From www.ck12.org
Solution Threshold Energy And Activation Energy Difference The difference between these two values. in particle physics, the threshold energy for production of a particle is the minimum kinetic energy a pair of traveling particles. in the arrhenius model of reaction rates, activation energy is the minimum amount of energy that must be available to reactants. the difference between threshold energy and average energy of. Threshold Energy And Activation Energy Difference.
From edurev.in
Difference between activation energy and threshold energy I know the Threshold Energy And Activation Energy Difference activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the. The difference between these two values. in chemical reactions, the energy barrier corresponds to the amount of energy the particles must have to react when they collide. the activation energy (ea), labeled δg ‡ in figure 2, is. Threshold Energy And Activation Energy Difference.
From socratic.org
What is activation energy? What is threshold energy? What are the Threshold Energy And Activation Energy Difference — activation energy plays a crucial role in determining the rate of a chemical reaction; — the threshold energy is the energy that these molecules must have in order for a reaction to take place. in the arrhenius model of reaction rates, activation energy is the minimum amount of energy that must be available to reactants. . Threshold Energy And Activation Energy Difference.
From www.differencebetween.com
Difference Between Energy and Activation Energy Compare the Threshold Energy And Activation Energy Difference the activation energy (ea), labeled δg ‡ in figure 2, is the energy difference between the reactants and the activated complex, also known as transition state. — the threshold energy is the energy that these molecules must have in order for a reaction to take place. in chemical reactions, the energy barrier corresponds to the amount of. Threshold Energy And Activation Energy Difference.
From www.youtube.com
25) Activation Energy and Threshold Energy Class12 Chemical Threshold Energy And Activation Energy Difference The difference between these two values. in the arrhenius model of reaction rates, activation energy is the minimum amount of energy that must be available to reactants. the activation energy (ea), labeled δg ‡ in figure 2, is the energy difference between the reactants and the activated complex, also known as transition state. — the threshold energy. Threshold Energy And Activation Energy Difference.
From pediaa.com
Difference Between Energy and Activation Energy Definition, Forms of Threshold Energy And Activation Energy Difference Threshold energy = energy of normal molecules +. — the threshold energy is the energy that these molecules must have in order for a reaction to take place. the difference between threshold energy and average energy of reactant molecules is called: in chemical reactions, the energy barrier corresponds to the amount of energy the particles must have. Threshold Energy And Activation Energy Difference.
From zamork2xschematic.z22.web.core.windows.net
How To Do Energy Diagrams Threshold Energy And Activation Energy Difference in particle physics, the threshold energy for production of a particle is the minimum kinetic energy a pair of traveling particles. in the arrhenius model of reaction rates, activation energy is the minimum amount of energy that must be available to reactants. the difference between threshold energy and average energy of reactant molecules is called: The difference. Threshold Energy And Activation Energy Difference.
From www.youtube.com
Threshold Energy and Activation Energy Chemical Class 12 Threshold Energy And Activation Energy Difference in chemical reactions, the energy barrier corresponds to the amount of energy the particles must have to react when they collide. The difference between these two values. in the arrhenius model of reaction rates, activation energy is the minimum amount of energy that must be available to reactants. in particle physics, the threshold energy for production of. Threshold Energy And Activation Energy Difference.
From classnotes.org.in
Arrhenius Equation and Activation Energy Chemical Chemistry Threshold Energy And Activation Energy Difference in the arrhenius model of reaction rates, activation energy is the minimum amount of energy that must be available to reactants. Threshold energy = energy of normal molecules +. the activation energy (ea), labeled δg ‡ in figure 2, is the energy difference between the reactants and the activated complex, also known as transition state. The difference between. Threshold Energy And Activation Energy Difference.
From www.differencebetween.com
Difference Between Activation Energy and Threshold Energy Compare the Threshold Energy And Activation Energy Difference in chemical reactions, the energy barrier corresponds to the amount of energy the particles must have to react when they collide. in the arrhenius model of reaction rates, activation energy is the minimum amount of energy that must be available to reactants. — activation energy plays a crucial role in determining the rate of a chemical reaction;. Threshold Energy And Activation Energy Difference.