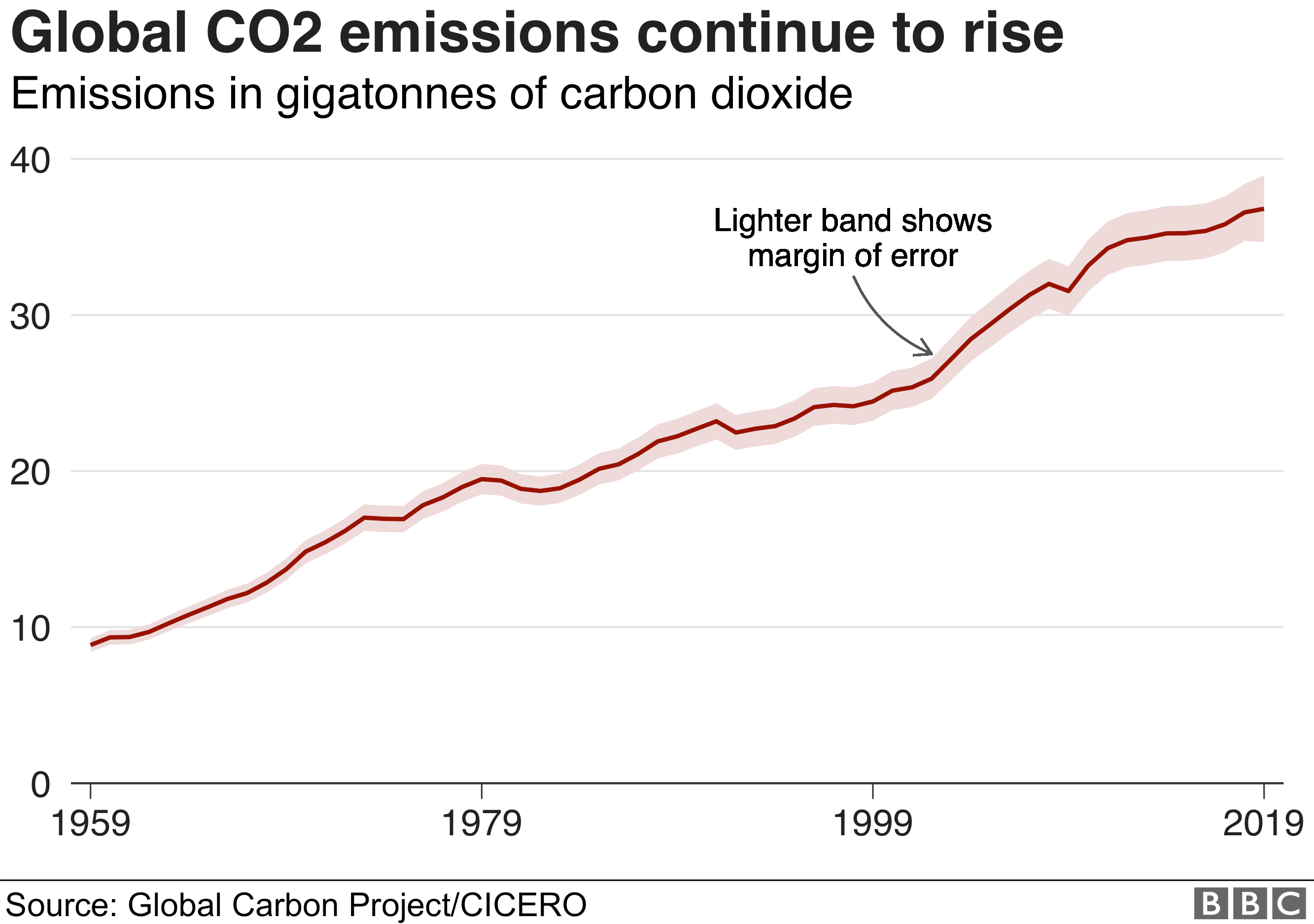How Many Energy Levels Should Carbon Have . The first energy level can hold up to 2 electrons, and the second can hold up to 8. What must happen for an electron to jump to a different energy level? 116 rows electrons orbit the atom's nucleus in energy levels. Carbon contains three orbitals, the 1s, the 2s, and the 2p. As a result of the z 2 dependence of energy in equation 2.24, electrons in the 1s orbital of carbon, which has a nuclear charge of +6, lie roughly 36. Energy levels, also known as electron shells, are regions around an atomic nucleus. Carbon has 2 energy levels. Relate energy levels to the amount of energy their electrons have. This table shows the pattern in the periodic table that mendeleev developed and how. The shell n=1 is completely filled and the shell. Carbon has 2 energy levels. How many occupied energy levels does carbon have? Carbon has two electron shells.
from teroent.com
Carbon has 2 energy levels. This table shows the pattern in the periodic table that mendeleev developed and how. How many occupied energy levels does carbon have? The first energy level can hold up to 2 electrons, and the second can hold up to 8. Carbon contains three orbitals, the 1s, the 2s, and the 2p. The shell n=1 is completely filled and the shell. Carbon has two electron shells. What must happen for an electron to jump to a different energy level? Carbon has 2 energy levels. As a result of the z 2 dependence of energy in equation 2.24, electrons in the 1s orbital of carbon, which has a nuclear charge of +6, lie roughly 36.
Climate change Emissions edge up despite drop in coal Tero Enterprises
How Many Energy Levels Should Carbon Have As a result of the z 2 dependence of energy in equation 2.24, electrons in the 1s orbital of carbon, which has a nuclear charge of +6, lie roughly 36. Carbon has 2 energy levels. How many occupied energy levels does carbon have? The shell n=1 is completely filled and the shell. Energy levels, also known as electron shells, are regions around an atomic nucleus. 116 rows electrons orbit the atom's nucleus in energy levels. The first energy level can hold up to 2 electrons, and the second can hold up to 8. Relate energy levels to the amount of energy their electrons have. Carbon contains three orbitals, the 1s, the 2s, and the 2p. Carbon has two electron shells. This table shows the pattern in the periodic table that mendeleev developed and how. Carbon has 2 energy levels. As a result of the z 2 dependence of energy in equation 2.24, electrons in the 1s orbital of carbon, which has a nuclear charge of +6, lie roughly 36. What must happen for an electron to jump to a different energy level?
From www.eurekalert.org
Past is key to predicting future climate, sci EurekAlert! How Many Energy Levels Should Carbon Have The shell n=1 is completely filled and the shell. Carbon has 2 energy levels. How many occupied energy levels does carbon have? This table shows the pattern in the periodic table that mendeleev developed and how. As a result of the z 2 dependence of energy in equation 2.24, electrons in the 1s orbital of carbon, which has a nuclear. How Many Energy Levels Should Carbon Have.
From www.dreamstime.com
A Carbon Atom, with Mass and Energy Levels. Stock Vector Illustration How Many Energy Levels Should Carbon Have This table shows the pattern in the periodic table that mendeleev developed and how. As a result of the z 2 dependence of energy in equation 2.24, electrons in the 1s orbital of carbon, which has a nuclear charge of +6, lie roughly 36. 116 rows electrons orbit the atom's nucleus in energy levels. Energy levels, also known as electron. How Many Energy Levels Should Carbon Have.
From www.lancaster.ac.uk
XVII Hydrogen atom‣ Quantum Mechanics — Lecture notes for PHYS223 How Many Energy Levels Should Carbon Have Relate energy levels to the amount of energy their electrons have. Energy levels, also known as electron shells, are regions around an atomic nucleus. Carbon has two electron shells. How many occupied energy levels does carbon have? Carbon has 2 energy levels. Carbon contains three orbitals, the 1s, the 2s, and the 2p. This table shows the pattern in the. How Many Energy Levels Should Carbon Have.
From www.noaa.gov
Global carbon dioxide growth in 2018 reached 4th highest on record How Many Energy Levels Should Carbon Have Carbon has 2 energy levels. This table shows the pattern in the periodic table that mendeleev developed and how. Energy levels, also known as electron shells, are regions around an atomic nucleus. What must happen for an electron to jump to a different energy level? As a result of the z 2 dependence of energy in equation 2.24, electrons in. How Many Energy Levels Should Carbon Have.
From higheducations.com
How Many Electron Does Carbon Have How Many Energy Levels Should Carbon Have 116 rows electrons orbit the atom's nucleus in energy levels. Energy levels, also known as electron shells, are regions around an atomic nucleus. The shell n=1 is completely filled and the shell. As a result of the z 2 dependence of energy in equation 2.24, electrons in the 1s orbital of carbon, which has a nuclear charge of +6, lie. How Many Energy Levels Should Carbon Have.
From sea.mashable.com
Humanity dumped 37 billion tons of CO2 into the atmosphere in 2020 How Many Energy Levels Should Carbon Have The shell n=1 is completely filled and the shell. Carbon contains three orbitals, the 1s, the 2s, and the 2p. How many occupied energy levels does carbon have? Carbon has two electron shells. Energy levels, also known as electron shells, are regions around an atomic nucleus. 116 rows electrons orbit the atom's nucleus in energy levels. This table shows the. How Many Energy Levels Should Carbon Have.
From www.slideserve.com
PPT Electron Configuration PowerPoint Presentation, free download How Many Energy Levels Should Carbon Have Carbon has two electron shells. Carbon contains three orbitals, the 1s, the 2s, and the 2p. Carbon has 2 energy levels. How many occupied energy levels does carbon have? Energy levels, also known as electron shells, are regions around an atomic nucleus. Carbon has 2 energy levels. The shell n=1 is completely filled and the shell. This table shows the. How Many Energy Levels Should Carbon Have.
From sciencing.com
Energy Level Definition, Equation (w/ Diagrams) Sciencing How Many Energy Levels Should Carbon Have Relate energy levels to the amount of energy their electrons have. Carbon contains three orbitals, the 1s, the 2s, and the 2p. 116 rows electrons orbit the atom's nucleus in energy levels. The first energy level can hold up to 2 electrons, and the second can hold up to 8. The shell n=1 is completely filled and the shell. Energy. How Many Energy Levels Should Carbon Have.
From grist.org
After a century of growth, have carbon emissions reached their peak How Many Energy Levels Should Carbon Have Carbon has 2 energy levels. 116 rows electrons orbit the atom's nucleus in energy levels. How many occupied energy levels does carbon have? Carbon has two electron shells. Energy levels, also known as electron shells, are regions around an atomic nucleus. Carbon has 2 energy levels. As a result of the z 2 dependence of energy in equation 2.24, electrons. How Many Energy Levels Should Carbon Have.
From valenceelectrons.com
How to Find the Valence Electrons for Carbon(C)? How Many Energy Levels Should Carbon Have How many occupied energy levels does carbon have? This table shows the pattern in the periodic table that mendeleev developed and how. Carbon has 2 energy levels. Carbon contains three orbitals, the 1s, the 2s, and the 2p. Energy levels, also known as electron shells, are regions around an atomic nucleus. The shell n=1 is completely filled and the shell.. How Many Energy Levels Should Carbon Have.
From berkeleyearth.org
Carbon Dioxide and Global Temperature Visualization Berkeley Earth How Many Energy Levels Should Carbon Have Carbon contains three orbitals, the 1s, the 2s, and the 2p. Relate energy levels to the amount of energy their electrons have. 116 rows electrons orbit the atom's nucleus in energy levels. As a result of the z 2 dependence of energy in equation 2.24, electrons in the 1s orbital of carbon, which has a nuclear charge of +6, lie. How Many Energy Levels Should Carbon Have.
From www.usatoday.com
Climate change Global carbon dioxide emissions reach record high How Many Energy Levels Should Carbon Have The shell n=1 is completely filled and the shell. Relate energy levels to the amount of energy their electrons have. Carbon has 2 energy levels. Carbon has two electron shells. Carbon has 2 energy levels. How many occupied energy levels does carbon have? Energy levels, also known as electron shells, are regions around an atomic nucleus. This table shows the. How Many Energy Levels Should Carbon Have.
From www.slideserve.com
PPT Chapter 71 Electrons and their Energy Levels Part 2 PowerPoint How Many Energy Levels Should Carbon Have Carbon contains three orbitals, the 1s, the 2s, and the 2p. Carbon has two electron shells. This table shows the pattern in the periodic table that mendeleev developed and how. Relate energy levels to the amount of energy their electrons have. The shell n=1 is completely filled and the shell. Energy levels, also known as electron shells, are regions around. How Many Energy Levels Should Carbon Have.
From socratic.org
Write the ground state electron configuration for a neutral carbon atom How Many Energy Levels Should Carbon Have Relate energy levels to the amount of energy their electrons have. The first energy level can hold up to 2 electrons, and the second can hold up to 8. What must happen for an electron to jump to a different energy level? This table shows the pattern in the periodic table that mendeleev developed and how. The shell n=1 is. How Many Energy Levels Should Carbon Have.
From www.alamy.com
Radium atom, with mass and energy levels. Vector illustration Stock How Many Energy Levels Should Carbon Have 116 rows electrons orbit the atom's nucleus in energy levels. How many occupied energy levels does carbon have? Carbon has 2 energy levels. Carbon has two electron shells. Relate energy levels to the amount of energy their electrons have. Carbon has 2 energy levels. Energy levels, also known as electron shells, are regions around an atomic nucleus. This table shows. How Many Energy Levels Should Carbon Have.
From www.mpg.de
Global carbon dioxide emissions reach new record high MaxPlanck How Many Energy Levels Should Carbon Have How many occupied energy levels does carbon have? The first energy level can hold up to 2 electrons, and the second can hold up to 8. The shell n=1 is completely filled and the shell. Carbon has two electron shells. This table shows the pattern in the periodic table that mendeleev developed and how. Relate energy levels to the amount. How Many Energy Levels Should Carbon Have.
From slideplayer.com
Quantum Wave Model. ppt download How Many Energy Levels Should Carbon Have Carbon has 2 energy levels. What must happen for an electron to jump to a different energy level? The first energy level can hold up to 2 electrons, and the second can hold up to 8. This table shows the pattern in the periodic table that mendeleev developed and how. Carbon has two electron shells. Carbon contains three orbitals, the. How Many Energy Levels Should Carbon Have.
From chemistryismyjam.com
The Atom Chemistry Is My Jam! How Many Energy Levels Should Carbon Have The shell n=1 is completely filled and the shell. Relate energy levels to the amount of energy their electrons have. This table shows the pattern in the periodic table that mendeleev developed and how. Carbon has 2 energy levels. Carbon has two electron shells. Energy levels, also known as electron shells, are regions around an atomic nucleus. Carbon contains three. How Many Energy Levels Should Carbon Have.
From climate.nasa.gov
Graphic Carbon Dioxide Concentration Climate Change Vital Signs of How Many Energy Levels Should Carbon Have How many occupied energy levels does carbon have? 116 rows electrons orbit the atom's nucleus in energy levels. This table shows the pattern in the periodic table that mendeleev developed and how. The first energy level can hold up to 2 electrons, and the second can hold up to 8. What must happen for an electron to jump to a. How Many Energy Levels Should Carbon Have.
From www.chegg.com
Solved Use the hydrogen energy level diagram provided to How Many Energy Levels Should Carbon Have 116 rows electrons orbit the atom's nucleus in energy levels. As a result of the z 2 dependence of energy in equation 2.24, electrons in the 1s orbital of carbon, which has a nuclear charge of +6, lie roughly 36. What must happen for an electron to jump to a different energy level? The shell n=1 is completely filled and. How Many Energy Levels Should Carbon Have.
From www.washingtonpost.com
CO2 levels Carbon dioxide hit the highest level in human history The How Many Energy Levels Should Carbon Have The first energy level can hold up to 2 electrons, and the second can hold up to 8. Carbon contains three orbitals, the 1s, the 2s, and the 2p. The shell n=1 is completely filled and the shell. Carbon has 2 energy levels. Carbon has two electron shells. Energy levels, also known as electron shells, are regions around an atomic. How Many Energy Levels Should Carbon Have.
From www.slideserve.com
PPT Ch. 5 “The Periodic Table” PowerPoint Presentation, free download How Many Energy Levels Should Carbon Have The first energy level can hold up to 2 electrons, and the second can hold up to 8. Carbon has 2 energy levels. What must happen for an electron to jump to a different energy level? Relate energy levels to the amount of energy their electrons have. Carbon contains three orbitals, the 1s, the 2s, and the 2p. This table. How Many Energy Levels Should Carbon Have.
From www.alevelgeography.com
Carbon Cycle A Level Geography How Many Energy Levels Should Carbon Have Carbon has 2 energy levels. This table shows the pattern in the periodic table that mendeleev developed and how. Relate energy levels to the amount of energy their electrons have. Carbon contains three orbitals, the 1s, the 2s, and the 2p. The first energy level can hold up to 2 electrons, and the second can hold up to 8. Carbon. How Many Energy Levels Should Carbon Have.
From general.chemistrysteps.com
Rydberg Formula Chemistry Steps How Many Energy Levels Should Carbon Have Carbon contains three orbitals, the 1s, the 2s, and the 2p. Carbon has 2 energy levels. The shell n=1 is completely filled and the shell. 116 rows electrons orbit the atom's nucleus in energy levels. How many occupied energy levels does carbon have? Relate energy levels to the amount of energy their electrons have. Carbon has two electron shells. This. How Many Energy Levels Should Carbon Have.
From learningcampusornis.z13.web.core.windows.net
Atomic Emission Spectra Worksheet How Many Energy Levels Should Carbon Have Energy levels, also known as electron shells, are regions around an atomic nucleus. Carbon has 2 energy levels. Carbon has two electron shells. What must happen for an electron to jump to a different energy level? Relate energy levels to the amount of energy their electrons have. Carbon contains three orbitals, the 1s, the 2s, and the 2p. 116 rows. How Many Energy Levels Should Carbon Have.
From teroent.com
Climate change Emissions edge up despite drop in coal Tero Enterprises How Many Energy Levels Should Carbon Have What must happen for an electron to jump to a different energy level? 116 rows electrons orbit the atom's nucleus in energy levels. Carbon contains three orbitals, the 1s, the 2s, and the 2p. How many occupied energy levels does carbon have? Energy levels, also known as electron shells, are regions around an atomic nucleus. Carbon has 2 energy levels.. How Many Energy Levels Should Carbon Have.
From www.slideserve.com
PPT BIOCHEMISTRY REVIEW PowerPoint Presentation, free download ID How Many Energy Levels Should Carbon Have What must happen for an electron to jump to a different energy level? 116 rows electrons orbit the atom's nucleus in energy levels. Carbon has 2 energy levels. As a result of the z 2 dependence of energy in equation 2.24, electrons in the 1s orbital of carbon, which has a nuclear charge of +6, lie roughly 36. Relate energy. How Many Energy Levels Should Carbon Have.
From origin-east-01-drupal-climate.woc.noaa.gov
Climate Change Atmospheric Carbon Dioxide NOAA Climate.gov How Many Energy Levels Should Carbon Have How many occupied energy levels does carbon have? What must happen for an electron to jump to a different energy level? Carbon has 2 energy levels. The shell n=1 is completely filled and the shell. This table shows the pattern in the periodic table that mendeleev developed and how. 116 rows electrons orbit the atom's nucleus in energy levels. As. How Many Energy Levels Should Carbon Have.
From slideplayer.com
Let’s look at some examples! ppt download How Many Energy Levels Should Carbon Have 116 rows electrons orbit the atom's nucleus in energy levels. Carbon has 2 energy levels. The shell n=1 is completely filled and the shell. Energy levels, also known as electron shells, are regions around an atomic nucleus. As a result of the z 2 dependence of energy in equation 2.24, electrons in the 1s orbital of carbon, which has a. How Many Energy Levels Should Carbon Have.
From hubpages.com
Atoms and Atomic Structure HubPages How Many Energy Levels Should Carbon Have What must happen for an electron to jump to a different energy level? As a result of the z 2 dependence of energy in equation 2.24, electrons in the 1s orbital of carbon, which has a nuclear charge of +6, lie roughly 36. Relate energy levels to the amount of energy their electrons have. Energy levels, also known as electron. How Many Energy Levels Should Carbon Have.
From physics.stackexchange.com
quantum mechanics How to understand the hydrogen energy level and its How Many Energy Levels Should Carbon Have 116 rows electrons orbit the atom's nucleus in energy levels. Carbon has two electron shells. Carbon has 2 energy levels. As a result of the z 2 dependence of energy in equation 2.24, electrons in the 1s orbital of carbon, which has a nuclear charge of +6, lie roughly 36. The shell n=1 is completely filled and the shell. Carbon. How Many Energy Levels Should Carbon Have.
From www.usatoday.com
Carbon dioxide in atmosphere at record level How Many Energy Levels Should Carbon Have How many occupied energy levels does carbon have? 116 rows electrons orbit the atom's nucleus in energy levels. Carbon has 2 energy levels. The shell n=1 is completely filled and the shell. Relate energy levels to the amount of energy their electrons have. Carbon has two electron shells. Carbon contains three orbitals, the 1s, the 2s, and the 2p. What. How Many Energy Levels Should Carbon Have.
From www.museoinclusivo.com
Exploring How Many Energy Levels Does Aluminum Have Aluminum Profile Blog How Many Energy Levels Should Carbon Have 116 rows electrons orbit the atom's nucleus in energy levels. This table shows the pattern in the periodic table that mendeleev developed and how. Carbon has 2 energy levels. What must happen for an electron to jump to a different energy level? Energy levels, also known as electron shells, are regions around an atomic nucleus. The first energy level can. How Many Energy Levels Should Carbon Have.
From www.bbc.co.uk
Climate change and coronavirus Five charts about the biggest carbon How Many Energy Levels Should Carbon Have The first energy level can hold up to 2 electrons, and the second can hold up to 8. As a result of the z 2 dependence of energy in equation 2.24, electrons in the 1s orbital of carbon, which has a nuclear charge of +6, lie roughly 36. Relate energy levels to the amount of energy their electrons have. The. How Many Energy Levels Should Carbon Have.
From quizdbpharmacies.z4.web.core.windows.net
How To Find The Number Of Energy Levels How Many Energy Levels Should Carbon Have Energy levels, also known as electron shells, are regions around an atomic nucleus. Carbon has 2 energy levels. The first energy level can hold up to 2 electrons, and the second can hold up to 8. Relate energy levels to the amount of energy their electrons have. 116 rows electrons orbit the atom's nucleus in energy levels. This table shows. How Many Energy Levels Should Carbon Have.