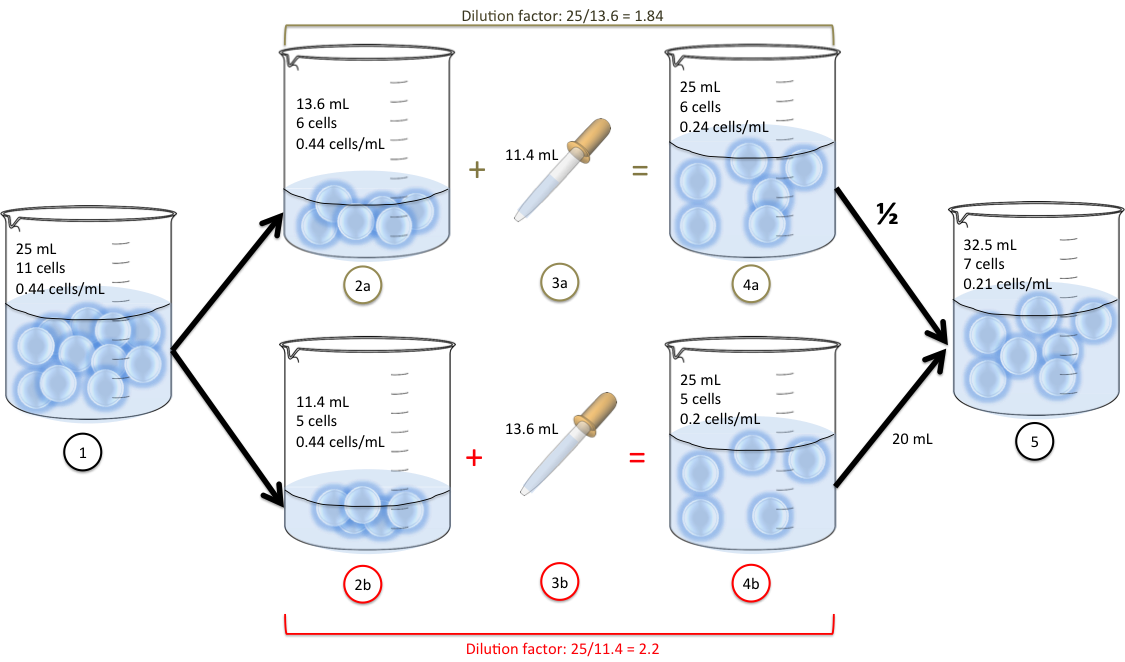
How To Use A Dilution Factor . To dilute a solution, you mix a small amount of strong solution with a lot of water or another liquid. This makes the solution weaker. For example, a 1:4 dilution ratio indicates 1 unit of solute and 4 units of solvent. Dilution factor is the factor by which the stock solution is diluted. The dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total solution after dilution. It may be expressed as the ratio of the volume of the final diluted solution. The dilution ratio tells us the amount of solute compared to the amount of solvent (s:d). The dilution factor is often used as the denominator of a fraction. It is often given as a ratio but can also be. Follow these five steps to make a dilution: For example, a df of 100 means a 1:100 dilution. If you do not know them already, use the dilution factor equation to.
from www.hemocytometer.org
To dilute a solution, you mix a small amount of strong solution with a lot of water or another liquid. The dilution factor is often used as the denominator of a fraction. For example, a 1:4 dilution ratio indicates 1 unit of solute and 4 units of solvent. It may be expressed as the ratio of the volume of the final diluted solution. For example, a df of 100 means a 1:100 dilution. This makes the solution weaker. The dilution ratio tells us the amount of solute compared to the amount of solvent (s:d). If you do not know them already, use the dilution factor equation to. It is often given as a ratio but can also be. Follow these five steps to make a dilution:
Using the dilution factor to calculate dilutions • Hemocytometer
How To Use A Dilution Factor To dilute a solution, you mix a small amount of strong solution with a lot of water or another liquid. The dilution ratio tells us the amount of solute compared to the amount of solvent (s:d). It may be expressed as the ratio of the volume of the final diluted solution. If you do not know them already, use the dilution factor equation to. For example, a df of 100 means a 1:100 dilution. The dilution factor is often used as the denominator of a fraction. The dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total solution after dilution. Dilution factor is the factor by which the stock solution is diluted. For example, a 1:4 dilution ratio indicates 1 unit of solute and 4 units of solvent. Follow these five steps to make a dilution: To dilute a solution, you mix a small amount of strong solution with a lot of water or another liquid. It is often given as a ratio but can also be. This makes the solution weaker.
From www.youtube.com
Serial Dilution Method Protocol Step Wise Explanation YouTube How To Use A Dilution Factor For example, a df of 100 means a 1:100 dilution. To dilute a solution, you mix a small amount of strong solution with a lot of water or another liquid. For example, a 1:4 dilution ratio indicates 1 unit of solute and 4 units of solvent. Dilution factor is the factor by which the stock solution is diluted. The dilution. How To Use A Dilution Factor.
From www.youtube.com
Dilution Chart.Helpful video. Understand how to prepare dilutions in How To Use A Dilution Factor To dilute a solution, you mix a small amount of strong solution with a lot of water or another liquid. The dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total solution after dilution. If you do not know them already, use the dilution factor equation to.. How To Use A Dilution Factor.
From www.youtube.com
Dilution Calculation Practice YouTube How To Use A Dilution Factor The dilution factor is often used as the denominator of a fraction. For example, a df of 100 means a 1:100 dilution. Follow these five steps to make a dilution: The dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total solution after dilution. It may be. How To Use A Dilution Factor.
From www.cierrescale.it
enclosure Full Less than dilution calculation Source Museum Immorality How To Use A Dilution Factor If you do not know them already, use the dilution factor equation to. It may be expressed as the ratio of the volume of the final diluted solution. For example, a 1:4 dilution ratio indicates 1 unit of solute and 4 units of solvent. Follow these five steps to make a dilution: For example, a df of 100 means a. How To Use A Dilution Factor.
From studyempathetic.z4.web.core.windows.net
Calculate Dilution Percent How To Use A Dilution Factor The dilution factor is often used as the denominator of a fraction. It is often given as a ratio but can also be. For example, a df of 100 means a 1:100 dilution. Dilution factor is the factor by which the stock solution is diluted. For example, a 1:4 dilution ratio indicates 1 unit of solute and 4 units of. How To Use A Dilution Factor.
From www.youtube.com
Calculating Dilution Factor YouTube How To Use A Dilution Factor It is often given as a ratio but can also be. For example, a df of 100 means a 1:100 dilution. This makes the solution weaker. If you do not know them already, use the dilution factor equation to. Dilution factor is the factor by which the stock solution is diluted. For example, a 1:4 dilution ratio indicates 1 unit. How To Use A Dilution Factor.
From sciencesavers.info
System, Calculator, Methodology, Makes use of, Examples sciencesavers How To Use A Dilution Factor Follow these five steps to make a dilution: Dilution factor is the factor by which the stock solution is diluted. The dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total solution after dilution. To dilute a solution, you mix a small amount of strong solution with. How To Use A Dilution Factor.
From microbeonline.com
Serial Dilution Method for Estimating Viable Count of Bacteria How To Use A Dilution Factor Follow these five steps to make a dilution: For example, a df of 100 means a 1:100 dilution. The dilution factor is often used as the denominator of a fraction. Dilution factor is the factor by which the stock solution is diluted. The dilution ratio tells us the amount of solute compared to the amount of solvent (s:d). If you. How To Use A Dilution Factor.
From socratic.org
How can I calculate the dilution factor using concentration? Socratic How To Use A Dilution Factor If you do not know them already, use the dilution factor equation to. For example, a df of 100 means a 1:100 dilution. For example, a 1:4 dilution ratio indicates 1 unit of solute and 4 units of solvent. Follow these five steps to make a dilution: The dilution factor is often used as the denominator of a fraction. The. How To Use A Dilution Factor.
From www.hemocytometer.org
Dilution factor calculator • Hemocytometer How To Use A Dilution Factor It is often given as a ratio but can also be. This makes the solution weaker. If you do not know them already, use the dilution factor equation to. The dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total solution after dilution. Dilution factor is the. How To Use A Dilution Factor.
From www.youtube.com
Dilution and Dilution Factor in Microbiology How to Calculate How To Use A Dilution Factor This makes the solution weaker. If you do not know them already, use the dilution factor equation to. For example, a df of 100 means a 1:100 dilution. The dilution ratio tells us the amount of solute compared to the amount of solvent (s:d). Follow these five steps to make a dilution: To dilute a solution, you mix a small. How To Use A Dilution Factor.
From www.carolina.com
Infographic—Lab Basics How to Perform Serial Dilutions How To Use A Dilution Factor It is often given as a ratio but can also be. For example, a 1:4 dilution ratio indicates 1 unit of solute and 4 units of solvent. The dilution factor is often used as the denominator of a fraction. Follow these five steps to make a dilution: To dilute a solution, you mix a small amount of strong solution with. How To Use A Dilution Factor.
From www.hemocytometer.org
Using the dilution factor to calculate dilutions • Hemocytometer How To Use A Dilution Factor The dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total solution after dilution. It is often given as a ratio but can also be. It may be expressed as the ratio of the volume of the final diluted solution. This makes the solution weaker. Follow these. How To Use A Dilution Factor.
From loelxqvfe.blob.core.windows.net
How To Calculate The Total Dilution Factor at Jason Briggs blog How To Use A Dilution Factor To dilute a solution, you mix a small amount of strong solution with a lot of water or another liquid. The dilution ratio tells us the amount of solute compared to the amount of solvent (s:d). The dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total. How To Use A Dilution Factor.
From loelxqvfe.blob.core.windows.net
How To Calculate The Total Dilution Factor at Jason Briggs blog How To Use A Dilution Factor For example, a df of 100 means a 1:100 dilution. The dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total solution after dilution. If you do not know them already, use the dilution factor equation to. It is often given as a ratio but can also. How To Use A Dilution Factor.
From exosmtljy.blob.core.windows.net
Dilution Cell Density at Bryan Owen blog How To Use A Dilution Factor It may be expressed as the ratio of the volume of the final diluted solution. The dilution ratio tells us the amount of solute compared to the amount of solvent (s:d). This makes the solution weaker. Dilution factor is the factor by which the stock solution is diluted. The dilution factor is often used as the denominator of a fraction.. How To Use A Dilution Factor.
From chem.libretexts.org
14.7 Solution Dilution Chemistry LibreTexts How To Use A Dilution Factor The dilution factor is often used as the denominator of a fraction. To dilute a solution, you mix a small amount of strong solution with a lot of water or another liquid. The dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total solution after dilution. For. How To Use A Dilution Factor.
From promolasopa510.weebly.com
Calculate dilution factor promolasopa How To Use A Dilution Factor For example, a 1:4 dilution ratio indicates 1 unit of solute and 4 units of solvent. This makes the solution weaker. To dilute a solution, you mix a small amount of strong solution with a lot of water or another liquid. It may be expressed as the ratio of the volume of the final diluted solution. Follow these five steps. How To Use A Dilution Factor.
From klaujfijv.blob.core.windows.net
How Do Dilutions Work at Michael Stanley blog How To Use A Dilution Factor It is often given as a ratio but can also be. The dilution factor is often used as the denominator of a fraction. The dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total solution after dilution. For example, a 1:4 dilution ratio indicates 1 unit of. How To Use A Dilution Factor.
From denisseqibowen.blogspot.com
How to Calculate Dilution Factor DenisseqiBowen How To Use A Dilution Factor This makes the solution weaker. The dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total solution after dilution. For example, a df of 100 means a 1:100 dilution. For example, a 1:4 dilution ratio indicates 1 unit of solute and 4 units of solvent. The dilution. How To Use A Dilution Factor.
From exyveacqb.blob.core.windows.net
Serial Dilution Questions Pharmacy at Bruce Guerra blog How To Use A Dilution Factor The dilution ratio tells us the amount of solute compared to the amount of solvent (s:d). It is often given as a ratio but can also be. This makes the solution weaker. Follow these five steps to make a dilution: If you do not know them already, use the dilution factor equation to. The dilution factor (or dilution ratio) is. How To Use A Dilution Factor.
From www.chegg.com
1a. Using the dilution series shown in the figure How To Use A Dilution Factor If you do not know them already, use the dilution factor equation to. For example, a 1:4 dilution ratio indicates 1 unit of solute and 4 units of solvent. Follow these five steps to make a dilution: This makes the solution weaker. Dilution factor is the factor by which the stock solution is diluted. The dilution factor (or dilution ratio). How To Use A Dilution Factor.
From klaunwhgn.blob.core.windows.net
How To Do Dilutions at Margaret Krueger blog How To Use A Dilution Factor If you do not know them already, use the dilution factor equation to. For example, a df of 100 means a 1:100 dilution. For example, a 1:4 dilution ratio indicates 1 unit of solute and 4 units of solvent. The dilution ratio tells us the amount of solute compared to the amount of solvent (s:d). This makes the solution weaker.. How To Use A Dilution Factor.
From joiuugyhn.blob.core.windows.net
Dilution Calculator Concentration at Bernard Butler blog How To Use A Dilution Factor If you do not know them already, use the dilution factor equation to. This makes the solution weaker. To dilute a solution, you mix a small amount of strong solution with a lot of water or another liquid. The dilution factor is often used as the denominator of a fraction. It is often given as a ratio but can also. How To Use A Dilution Factor.
From www.hemocytometer.org
Using the dilution factor to calculate dilutions • Hemocytometer How To Use A Dilution Factor This makes the solution weaker. For example, a 1:4 dilution ratio indicates 1 unit of solute and 4 units of solvent. For example, a df of 100 means a 1:100 dilution. If you do not know them already, use the dilution factor equation to. The dilution factor (or dilution ratio) is the notation used to express how much of the. How To Use A Dilution Factor.
From coffeenatics.com
How Much Caffeine is in McDonald's Iced Coffee? Discover the Buzz How To Use A Dilution Factor Dilution factor is the factor by which the stock solution is diluted. To dilute a solution, you mix a small amount of strong solution with a lot of water or another liquid. For example, a df of 100 means a 1:100 dilution. Follow these five steps to make a dilution: The dilution factor (or dilution ratio) is the notation used. How To Use A Dilution Factor.
From www.chegg.com
Solved A tenfold serial dilution of a microbial sample How To Use A Dilution Factor The dilution ratio tells us the amount of solute compared to the amount of solvent (s:d). It may be expressed as the ratio of the volume of the final diluted solution. For example, a 1:4 dilution ratio indicates 1 unit of solute and 4 units of solvent. For example, a df of 100 means a 1:100 dilution. The dilution factor. How To Use A Dilution Factor.
From www.youtube.com
How to Calculate Dilution Factor YouTube How To Use A Dilution Factor For example, a df of 100 means a 1:100 dilution. Follow these five steps to make a dilution: For example, a 1:4 dilution ratio indicates 1 unit of solute and 4 units of solvent. This makes the solution weaker. The dilution ratio tells us the amount of solute compared to the amount of solvent (s:d). The dilution factor (or dilution. How To Use A Dilution Factor.
From studyempathetic.z4.web.core.windows.net
Calculate Dilution Percent How To Use A Dilution Factor The dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total solution after dilution. Follow these five steps to make a dilution: For example, a df of 100 means a 1:100 dilution. To dilute a solution, you mix a small amount of strong solution with a lot. How To Use A Dilution Factor.
From www.chegg.com
Due Submit to the GTA at the beginning of your next How To Use A Dilution Factor The dilution factor is often used as the denominator of a fraction. The dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total solution after dilution. For example, a df of 100 means a 1:100 dilution. For example, a 1:4 dilution ratio indicates 1 unit of solute. How To Use A Dilution Factor.
From admissions.liba.edu
2024’s Top 5 Tips How to Use Apple Cider Vinegar for a Tighter Jawline How To Use A Dilution Factor For example, a df of 100 means a 1:100 dilution. To dilute a solution, you mix a small amount of strong solution with a lot of water or another liquid. The dilution factor is often used as the denominator of a fraction. For example, a 1:4 dilution ratio indicates 1 unit of solute and 4 units of solvent. The dilution. How To Use A Dilution Factor.
From www.slideserve.com
PPT Pharmaceutical Calculations (5) PowerPoint Presentation, free How To Use A Dilution Factor This makes the solution weaker. The dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total solution after dilution. For example, a 1:4 dilution ratio indicates 1 unit of solute and 4 units of solvent. It is often given as a ratio but can also be. To. How To Use A Dilution Factor.
From microbeonline.com
Serial Dilution Method for Estimating Viable Count of Bacteria How To Use A Dilution Factor The dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total solution after dilution. For example, a 1:4 dilution ratio indicates 1 unit of solute and 4 units of solvent. It is often given as a ratio but can also be. To dilute a solution, you mix. How To Use A Dilution Factor.
From studyempathetic.z4.web.core.windows.net
Calculate Dilution Percent How To Use A Dilution Factor The dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total solution after dilution. The dilution ratio tells us the amount of solute compared to the amount of solvent (s:d). This makes the solution weaker. To dilute a solution, you mix a small amount of strong solution. How To Use A Dilution Factor.
From ar.inspiredpencil.com
Serial Dilution Diagram How To Use A Dilution Factor Dilution factor is the factor by which the stock solution is diluted. Follow these five steps to make a dilution: For example, a 1:4 dilution ratio indicates 1 unit of solute and 4 units of solvent. It is often given as a ratio but can also be. To dilute a solution, you mix a small amount of strong solution with. How To Use A Dilution Factor.