Purpose Of Electrode In Galvanic Cell . In writing the equations, it is. In writing the equations, it is. The galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from the. In a galvanic cell, it is the positive electrode, as. In an electrolytic cell (right), an external source of electrical energy is used to generate a potential difference between the electrodes that forces electrons to flow, driving a. The cathode is the electrode where reduction (gain of electrons) takes place (metal b electrode);
from www.scienceabc.com
The galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from the. In a galvanic cell, it is the positive electrode, as. In writing the equations, it is. The cathode is the electrode where reduction (gain of electrons) takes place (metal b electrode); In writing the equations, it is. In an electrolytic cell (right), an external source of electrical energy is used to generate a potential difference between the electrodes that forces electrons to flow, driving a.
Galvanic Cell Definition, Diagram And Working
Purpose Of Electrode In Galvanic Cell In writing the equations, it is. In writing the equations, it is. The galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from the. In writing the equations, it is. The cathode is the electrode where reduction (gain of electrons) takes place (metal b electrode); In a galvanic cell, it is the positive electrode, as. In an electrolytic cell (right), an external source of electrical energy is used to generate a potential difference between the electrodes that forces electrons to flow, driving a.
From www.chegg.com
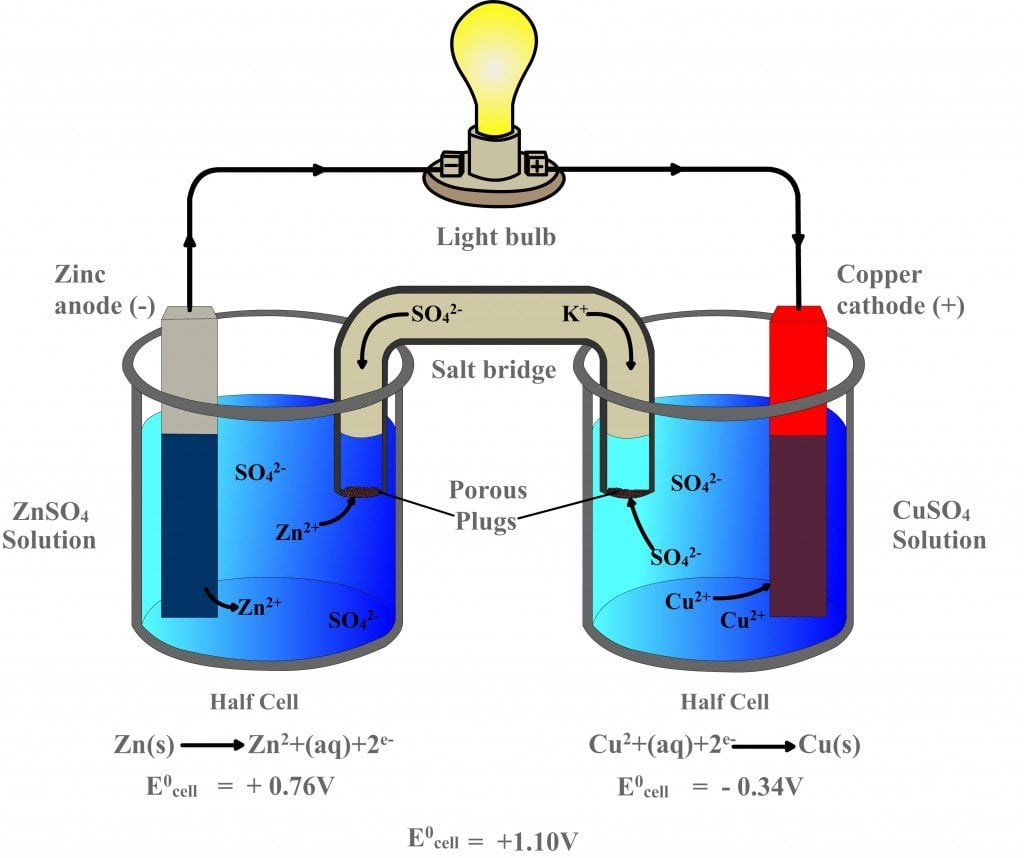
Solved A galvanic cell is set up under standard conditions Purpose Of Electrode In Galvanic Cell In a galvanic cell, it is the positive electrode, as. In writing the equations, it is. The galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from the. The cathode is the electrode where reduction (gain of electrons) takes place (metal b electrode); In an electrolytic cell (right), an external source. Purpose Of Electrode In Galvanic Cell.
From webapi.bu.edu
⭐ Difference between galvanic cell and daniell cell. Difference Between Purpose Of Electrode In Galvanic Cell In an electrolytic cell (right), an external source of electrical energy is used to generate a potential difference between the electrodes that forces electrons to flow, driving a. The galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from the. In a galvanic cell, it is the positive electrode, as. In. Purpose Of Electrode In Galvanic Cell.
From medium.com
Galvanic Cells. In the world of electrochemistry… by Abdullah Medium Purpose Of Electrode In Galvanic Cell In writing the equations, it is. The cathode is the electrode where reduction (gain of electrons) takes place (metal b electrode); In an electrolytic cell (right), an external source of electrical energy is used to generate a potential difference between the electrodes that forces electrons to flow, driving a. In writing the equations, it is. In a galvanic cell, it. Purpose Of Electrode In Galvanic Cell.
From www.coursehero.com
[Solved] draw a galvanic cell and label the anode/cathode, cell Purpose Of Electrode In Galvanic Cell In a galvanic cell, it is the positive electrode, as. In an electrolytic cell (right), an external source of electrical energy is used to generate a potential difference between the electrodes that forces electrons to flow, driving a. The galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from the. In. Purpose Of Electrode In Galvanic Cell.
From www.slideserve.com
PPT Galvanic Cells Converting chemical energy to electrical energy Purpose Of Electrode In Galvanic Cell The cathode is the electrode where reduction (gain of electrons) takes place (metal b electrode); The galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from the. In writing the equations, it is. In writing the equations, it is. In a galvanic cell, it is the positive electrode, as. In an. Purpose Of Electrode In Galvanic Cell.
From www.slideserve.com
PPT Voltaic/Galvanic Cells PowerPoint Presentation, free download Purpose Of Electrode In Galvanic Cell In writing the equations, it is. In writing the equations, it is. In a galvanic cell, it is the positive electrode, as. The galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from the. In an electrolytic cell (right), an external source of electrical energy is used to generate a potential. Purpose Of Electrode In Galvanic Cell.
From chem.libretexts.org
1 Electrochemical Cells (Experiment) Chemistry LibreTexts Purpose Of Electrode In Galvanic Cell In writing the equations, it is. In a galvanic cell, it is the positive electrode, as. The cathode is the electrode where reduction (gain of electrons) takes place (metal b electrode); The galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from the. In an electrolytic cell (right), an external source. Purpose Of Electrode In Galvanic Cell.
From courses.lumenlearning.com
Galvanic Cells Chemistry Purpose Of Electrode In Galvanic Cell The cathode is the electrode where reduction (gain of electrons) takes place (metal b electrode); In writing the equations, it is. In an electrolytic cell (right), an external source of electrical energy is used to generate a potential difference between the electrodes that forces electrons to flow, driving a. In a galvanic cell, it is the positive electrode, as. In. Purpose Of Electrode In Galvanic Cell.
From www.vrogue.co
Galvanic Cell Animation Galvanic Cell Cell Electrical vrogue.co Purpose Of Electrode In Galvanic Cell In writing the equations, it is. In writing the equations, it is. In a galvanic cell, it is the positive electrode, as. In an electrolytic cell (right), an external source of electrical energy is used to generate a potential difference between the electrodes that forces electrons to flow, driving a. The galvanic cell, or called voltaic cell, is an electrochemical. Purpose Of Electrode In Galvanic Cell.
From www.vecteezy.com
Voltaic galvanic cell or daniell cell.Redox reaction.Oxidation and Purpose Of Electrode In Galvanic Cell The galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from the. In writing the equations, it is. In a galvanic cell, it is the positive electrode, as. In writing the equations, it is. The cathode is the electrode where reduction (gain of electrons) takes place (metal b electrode); In an. Purpose Of Electrode In Galvanic Cell.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID5404890 Purpose Of Electrode In Galvanic Cell In writing the equations, it is. The cathode is the electrode where reduction (gain of electrons) takes place (metal b electrode); In a galvanic cell, it is the positive electrode, as. In writing the equations, it is. In an electrolytic cell (right), an external source of electrical energy is used to generate a potential difference between the electrodes that forces. Purpose Of Electrode In Galvanic Cell.
From keyesvannuyshyundai.blogspot.com
draw the diagram of electrolytic cell and explain keyesvannuyshyundai Purpose Of Electrode In Galvanic Cell In writing the equations, it is. In an electrolytic cell (right), an external source of electrical energy is used to generate a potential difference between the electrodes that forces electrons to flow, driving a. In writing the equations, it is. In a galvanic cell, it is the positive electrode, as. The galvanic cell, or called voltaic cell, is an electrochemical. Purpose Of Electrode In Galvanic Cell.
From ck12.org
In the electrochemical cell illustrated above, the copper metal must be Purpose Of Electrode In Galvanic Cell The galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from the. In writing the equations, it is. In writing the equations, it is. In an electrolytic cell (right), an external source of electrical energy is used to generate a potential difference between the electrodes that forces electrons to flow, driving. Purpose Of Electrode In Galvanic Cell.
From mavink.com
Diagram Of Electrochemical Cell Purpose Of Electrode In Galvanic Cell In an electrolytic cell (right), an external source of electrical energy is used to generate a potential difference between the electrodes that forces electrons to flow, driving a. In writing the equations, it is. In writing the equations, it is. The galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. Purpose Of Electrode In Galvanic Cell.
From www.pinterest.com
Understanding purpose of salt bridge Galvanic cell, Electrochemical Purpose Of Electrode In Galvanic Cell In a galvanic cell, it is the positive electrode, as. In writing the equations, it is. The cathode is the electrode where reduction (gain of electrons) takes place (metal b electrode); In an electrolytic cell (right), an external source of electrical energy is used to generate a potential difference between the electrodes that forces electrons to flow, driving a. In. Purpose Of Electrode In Galvanic Cell.
From wisc.pb.unizin.org
D39.4 Introduction to Voltaic Cells Chemistry 109 Fall 2021 Purpose Of Electrode In Galvanic Cell In writing the equations, it is. In a galvanic cell, it is the positive electrode, as. The galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from the. In an electrolytic cell (right), an external source of electrical energy is used to generate a potential difference between the electrodes that forces. Purpose Of Electrode In Galvanic Cell.
From chem.libretexts.org
Chapter 19.1 Describing Electrochemical Cells Chemistry LibreTexts Purpose Of Electrode In Galvanic Cell The cathode is the electrode where reduction (gain of electrons) takes place (metal b electrode); In writing the equations, it is. In an electrolytic cell (right), an external source of electrical energy is used to generate a potential difference between the electrodes that forces electrons to flow, driving a. The galvanic cell, or called voltaic cell, is an electrochemical cell. Purpose Of Electrode In Galvanic Cell.
From socratic.org
Two halfcells in a galvanic cell consist of one iron (Fe(s)) electrode Purpose Of Electrode In Galvanic Cell In a galvanic cell, it is the positive electrode, as. In writing the equations, it is. In writing the equations, it is. The cathode is the electrode where reduction (gain of electrons) takes place (metal b electrode); The galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from the. In an. Purpose Of Electrode In Galvanic Cell.
From in.pinterest.com
three different types of electronic devices are shown in this diagram Purpose Of Electrode In Galvanic Cell The cathode is the electrode where reduction (gain of electrons) takes place (metal b electrode); In a galvanic cell, it is the positive electrode, as. The galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from the. In an electrolytic cell (right), an external source of electrical energy is used to. Purpose Of Electrode In Galvanic Cell.
From www.scienceabc.com
Galvanic Cell Definition, Diagram And Working Purpose Of Electrode In Galvanic Cell In an electrolytic cell (right), an external source of electrical energy is used to generate a potential difference between the electrodes that forces electrons to flow, driving a. In a galvanic cell, it is the positive electrode, as. The cathode is the electrode where reduction (gain of electrons) takes place (metal b electrode); In writing the equations, it is. The. Purpose Of Electrode In Galvanic Cell.
From www.differencebetween.com
Difference Between Galvanic Cell and Concentration Cell Compare the Purpose Of Electrode In Galvanic Cell In a galvanic cell, it is the positive electrode, as. In an electrolytic cell (right), an external source of electrical energy is used to generate a potential difference between the electrodes that forces electrons to flow, driving a. The galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from the. The. Purpose Of Electrode In Galvanic Cell.
From www.vecteezy.com
Electrochemical cell or Galvanic cell with Voltmeter and the function Purpose Of Electrode In Galvanic Cell The galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from the. In an electrolytic cell (right), an external source of electrical energy is used to generate a potential difference between the electrodes that forces electrons to flow, driving a. In writing the equations, it is. In a galvanic cell, it. Purpose Of Electrode In Galvanic Cell.
From scienceinfo.com
Galvanic Cell (Voltaic Cell) Definition, Working Principle Purpose Of Electrode In Galvanic Cell In writing the equations, it is. In writing the equations, it is. In a galvanic cell, it is the positive electrode, as. In an electrolytic cell (right), an external source of electrical energy is used to generate a potential difference between the electrodes that forces electrons to flow, driving a. The galvanic cell, or called voltaic cell, is an electrochemical. Purpose Of Electrode In Galvanic Cell.
From www.echemi.com
Where do negative ions migrate from the salt bridge to which electrode Purpose Of Electrode In Galvanic Cell In an electrolytic cell (right), an external source of electrical energy is used to generate a potential difference between the electrodes that forces electrons to flow, driving a. In writing the equations, it is. In writing the equations, it is. The galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. Purpose Of Electrode In Galvanic Cell.
From www.slideserve.com
PPT Galvanic Cells PowerPoint Presentation, free download ID3222668 Purpose Of Electrode In Galvanic Cell The galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from the. In a galvanic cell, it is the positive electrode, as. The cathode is the electrode where reduction (gain of electrons) takes place (metal b electrode); In writing the equations, it is. In writing the equations, it is. In an. Purpose Of Electrode In Galvanic Cell.
From www.vrogue.co
Difference Between Galvanic Cells And Electrolytic Ce vrogue.co Purpose Of Electrode In Galvanic Cell In writing the equations, it is. The cathode is the electrode where reduction (gain of electrons) takes place (metal b electrode); In an electrolytic cell (right), an external source of electrical energy is used to generate a potential difference between the electrodes that forces electrons to flow, driving a. In writing the equations, it is. In a galvanic cell, it. Purpose Of Electrode In Galvanic Cell.
From chem.libretexts.org
11.1 Galvanic Cells Chemistry LibreTexts Purpose Of Electrode In Galvanic Cell The cathode is the electrode where reduction (gain of electrons) takes place (metal b electrode); The galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from the. In an electrolytic cell (right), an external source of electrical energy is used to generate a potential difference between the electrodes that forces electrons. Purpose Of Electrode In Galvanic Cell.
From chem.libretexts.org
11.1 Galvanic Cells Chemistry LibreTexts Purpose Of Electrode In Galvanic Cell The galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from the. In an electrolytic cell (right), an external source of electrical energy is used to generate a potential difference between the electrodes that forces electrons to flow, driving a. In writing the equations, it is. In a galvanic cell, it. Purpose Of Electrode In Galvanic Cell.
From sites.google.com
Electrochemistry engineering chemistry Purpose Of Electrode In Galvanic Cell The galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from the. In an electrolytic cell (right), an external source of electrical energy is used to generate a potential difference between the electrodes that forces electrons to flow, driving a. The cathode is the electrode where reduction (gain of electrons) takes. Purpose Of Electrode In Galvanic Cell.
From schoolbag.info
Electrochemical Cells Electrochemistry Training MCAT General Purpose Of Electrode In Galvanic Cell In a galvanic cell, it is the positive electrode, as. The cathode is the electrode where reduction (gain of electrons) takes place (metal b electrode); In writing the equations, it is. In an electrolytic cell (right), an external source of electrical energy is used to generate a potential difference between the electrodes that forces electrons to flow, driving a. In. Purpose Of Electrode In Galvanic Cell.
From www.geeksforgeeks.org
Electrochemical Cell Definition, Types, Working Principle & Uses Purpose Of Electrode In Galvanic Cell In writing the equations, it is. In writing the equations, it is. The cathode is the electrode where reduction (gain of electrons) takes place (metal b electrode); In an electrolytic cell (right), an external source of electrical energy is used to generate a potential difference between the electrodes that forces electrons to flow, driving a. In a galvanic cell, it. Purpose Of Electrode In Galvanic Cell.
From www.youtube.com
Galvanic Cell Definition, Construction, Working, Example, Diagram Purpose Of Electrode In Galvanic Cell The galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from the. In an electrolytic cell (right), an external source of electrical energy is used to generate a potential difference between the electrodes that forces electrons to flow, driving a. The cathode is the electrode where reduction (gain of electrons) takes. Purpose Of Electrode In Galvanic Cell.
From www.dreamstime.com
Simple Electrochemical or Galvanic Cell. the Daniell Cell. Stock Vector Purpose Of Electrode In Galvanic Cell The cathode is the electrode where reduction (gain of electrons) takes place (metal b electrode); In an electrolytic cell (right), an external source of electrical energy is used to generate a potential difference between the electrodes that forces electrons to flow, driving a. In writing the equations, it is. In writing the equations, it is. The galvanic cell, or called. Purpose Of Electrode In Galvanic Cell.
From www.jove.com
Voltaic/Galvanic Cells Principle, Components, Cell Notation (Video) JoVE Purpose Of Electrode In Galvanic Cell In writing the equations, it is. The cathode is the electrode where reduction (gain of electrons) takes place (metal b electrode); In writing the equations, it is. In an electrolytic cell (right), an external source of electrical energy is used to generate a potential difference between the electrodes that forces electrons to flow, driving a. In a galvanic cell, it. Purpose Of Electrode In Galvanic Cell.
From www.youtube.com
Ch. 203 Inert Electrodes/Voltaic Cells YouTube Purpose Of Electrode In Galvanic Cell In an electrolytic cell (right), an external source of electrical energy is used to generate a potential difference between the electrodes that forces electrons to flow, driving a. In writing the equations, it is. The cathode is the electrode where reduction (gain of electrons) takes place (metal b electrode); In a galvanic cell, it is the positive electrode, as. The. Purpose Of Electrode In Galvanic Cell.