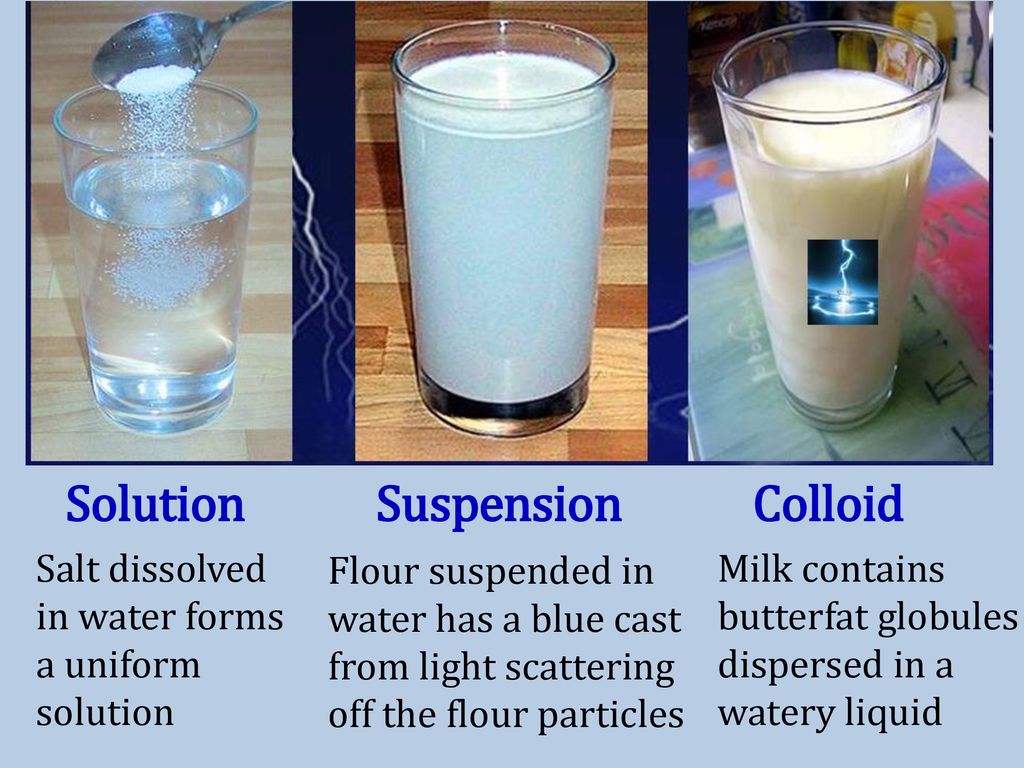
Suspension Colloid And Emulsion . A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a. Updated on october 29, 2019. B) the particles of a suspension will. An emulsion is a mixture of two. The substance that is dissolved is the solute. A suspension is a mixture of particles with diameters of about 1 µm (1000 nm) that are distributed throughout a second phase. In summary, the key difference between solution suspension and emulsion is that a solution is a mixture of two and suspension is a heterogeneous. Solutions, suspensions, colloids, and other dispersions are similar but have characteristics that set each one apart from the others. A solution is a homogeneous mixture of two or more components. What factor distinguishes a suspension from a colloid? The dissolving agent is the solvent. Common suspensions include paint, blood, and hot. A) light reflects off the particles of a suspension. An emulsion, on the other hand, is a specific type of colloid where the dispersed phase consists of tiny droplets of one liquid suspended in another. The size of particles in colloids is larger than in solution but smaller than that of suspensions.
from slideplayer.com
What factor distinguishes a suspension from a colloid? Common suspensions include paint, blood, and hot. Updated on october 29, 2019. An emulsion is a mixture of two. A suspension is a mixture of particles with diameters of about 1 µm (1000 nm) that are distributed throughout a second phase. In summary, the key difference between solution suspension and emulsion is that a solution is a mixture of two and suspension is a heterogeneous. B) the particles of a suspension will. The size of particles in colloids is larger than in solution but smaller than that of suspensions. A solution is a homogeneous mixture of two or more components. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a.
Solutions, Colloids, and Suspensions ppt download
Suspension Colloid And Emulsion A solution is a homogeneous mixture of two or more components. A solution is a homogeneous mixture of two or more components. The dissolving agent is the solvent. A suspension is a mixture of particles with diameters of about 1 µm (1000 nm) that are distributed throughout a second phase. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a. An emulsion, on the other hand, is a specific type of colloid where the dispersed phase consists of tiny droplets of one liquid suspended in another. Solutions, suspensions, colloids, and other dispersions are similar but have characteristics that set each one apart from the others. A) light reflects off the particles of a suspension. The size of particles in colloids is larger than in solution but smaller than that of suspensions. Updated on october 29, 2019. Common suspensions include paint, blood, and hot. B) the particles of a suspension will. An emulsion is a mixture of two. The substance that is dissolved is the solute. What factor distinguishes a suspension from a colloid? In summary, the key difference between solution suspension and emulsion is that a solution is a mixture of two and suspension is a heterogeneous.
From studylib.net
Solutions, Colloids, Suspension Suspension Colloid And Emulsion B) the particles of a suspension will. A solution is a homogeneous mixture of two or more components. The size of particles in colloids is larger than in solution but smaller than that of suspensions. Updated on october 29, 2019. Solutions, suspensions, colloids, and other dispersions are similar but have characteristics that set each one apart from the others. A). Suspension Colloid And Emulsion.
From www.differencebetween.com
Difference Between Colloid and Emulsion Compare the Difference Suspension Colloid And Emulsion A solution is a homogeneous mixture of two or more components. The size of particles in colloids is larger than in solution but smaller than that of suspensions. The dissolving agent is the solvent. B) the particles of a suspension will. Solutions, suspensions, colloids, and other dispersions are similar but have characteristics that set each one apart from the others.. Suspension Colloid And Emulsion.
From www.britannica.com
Emulsion polymerization chemistry Britannica Suspension Colloid And Emulsion A) light reflects off the particles of a suspension. B) the particles of a suspension will. Updated on october 29, 2019. The dissolving agent is the solvent. Solutions, suspensions, colloids, and other dispersions are similar but have characteristics that set each one apart from the others. Common suspensions include paint, blood, and hot. A colloid is a heterogeneous mixture in. Suspension Colloid And Emulsion.
From www.slideserve.com
PPT UNIT 10 SOLUTIONS TEST on 5/1/14 PowerPoint Presentation, free Suspension Colloid And Emulsion The substance that is dissolved is the solute. An emulsion is a mixture of two. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a. The size of particles in colloids is larger than in solution but smaller than that of suspensions. A solution is a homogeneous mixture. Suspension Colloid And Emulsion.
From www.majordifferences.com
Difference Between True Solutions, Colloidal solution and Suspension Suspension Colloid And Emulsion The size of particles in colloids is larger than in solution but smaller than that of suspensions. An emulsion, on the other hand, is a specific type of colloid where the dispersed phase consists of tiny droplets of one liquid suspended in another. A suspension is a mixture of particles with diameters of about 1 µm (1000 nm) that are. Suspension Colloid And Emulsion.
From www.youtube.com
L9 APPLICATION OF COLLOID AND EMULSION YouTube Suspension Colloid And Emulsion The size of particles in colloids is larger than in solution but smaller than that of suspensions. B) the particles of a suspension will. The dissolving agent is the solvent. In summary, the key difference between solution suspension and emulsion is that a solution is a mixture of two and suspension is a heterogeneous. An emulsion is a mixture of. Suspension Colloid And Emulsion.
From pediaa.com
Difference Between Colloid and Suspension Definition, Properties Suspension Colloid And Emulsion Common suspensions include paint, blood, and hot. In summary, the key difference between solution suspension and emulsion is that a solution is a mixture of two and suspension is a heterogeneous. An emulsion, on the other hand, is a specific type of colloid where the dispersed phase consists of tiny droplets of one liquid suspended in another. Updated on october. Suspension Colloid And Emulsion.
From sciencenotes.org
What Is a Colloid? Definition and Examples Suspension Colloid And Emulsion The size of particles in colloids is larger than in solution but smaller than that of suspensions. Updated on october 29, 2019. Common suspensions include paint, blood, and hot. What factor distinguishes a suspension from a colloid? An emulsion, on the other hand, is a specific type of colloid where the dispersed phase consists of tiny droplets of one liquid. Suspension Colloid And Emulsion.
From byjus.com
Suspensions & Colloids Difference Between Colloid & SuspensionByju's Suspension Colloid And Emulsion B) the particles of a suspension will. A solution is a homogeneous mixture of two or more components. In summary, the key difference between solution suspension and emulsion is that a solution is a mixture of two and suspension is a heterogeneous. The substance that is dissolved is the solute. A) light reflects off the particles of a suspension. Updated. Suspension Colloid And Emulsion.
From www.geeksforgeeks.org
Suspension(Chemistry) Definition, Properties, Examples, and FAQs Suspension Colloid And Emulsion B) the particles of a suspension will. In summary, the key difference between solution suspension and emulsion is that a solution is a mixture of two and suspension is a heterogeneous. An emulsion, on the other hand, is a specific type of colloid where the dispersed phase consists of tiny droplets of one liquid suspended in another. What factor distinguishes. Suspension Colloid And Emulsion.
From www.numerade.com
SOLVED Classify each of these simple solutions as solution, suspension Suspension Colloid And Emulsion An emulsion, on the other hand, is a specific type of colloid where the dispersed phase consists of tiny droplets of one liquid suspended in another. An emulsion is a mixture of two. Solutions, suspensions, colloids, and other dispersions are similar but have characteristics that set each one apart from the others. Updated on october 29, 2019. A suspension is. Suspension Colloid And Emulsion.
From www.youtube.com
Colloids and Emulsions Mr H YouTube Suspension Colloid And Emulsion A solution is a homogeneous mixture of two or more components. The dissolving agent is the solvent. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a. Solutions, suspensions, colloids, and other dispersions are similar but have characteristics that set each one apart from the others. The substance. Suspension Colloid And Emulsion.
From in.pinterest.com
Solutions, suspensions, and colloids Suspension mixture, Body diagram Suspension Colloid And Emulsion The size of particles in colloids is larger than in solution but smaller than that of suspensions. A) light reflects off the particles of a suspension. B) the particles of a suspension will. An emulsion is a mixture of two. Common suspensions include paint, blood, and hot. A suspension is a mixture of particles with diameters of about 1 µm. Suspension Colloid And Emulsion.
From slideplayer.com
Solutions, Colloids, and Suspensions ppt download Suspension Colloid And Emulsion Common suspensions include paint, blood, and hot. An emulsion, on the other hand, is a specific type of colloid where the dispersed phase consists of tiny droplets of one liquid suspended in another. Updated on october 29, 2019. The substance that is dissolved is the solute. What factor distinguishes a suspension from a colloid? A solution is a homogeneous mixture. Suspension Colloid And Emulsion.
From quizizz.com
Solution, Colloid and Suspension Science Quizizz Suspension Colloid And Emulsion B) the particles of a suspension will. An emulsion, on the other hand, is a specific type of colloid where the dispersed phase consists of tiny droplets of one liquid suspended in another. Common suspensions include paint, blood, and hot. A suspension is a mixture of particles with diameters of about 1 µm (1000 nm) that are distributed throughout a. Suspension Colloid And Emulsion.
From www.myshared.ru
Презентация на тему "Colloidal systems. Classes of solution True Suspension Colloid And Emulsion Solutions, suspensions, colloids, and other dispersions are similar but have characteristics that set each one apart from the others. An emulsion is a mixture of two. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a. The substance that is dissolved is the solute. An emulsion, on the. Suspension Colloid And Emulsion.
From byjus.com
Suspensions (Chemistry) Definition, Properties, Examples with Videos Suspension Colloid And Emulsion A suspension is a mixture of particles with diameters of about 1 µm (1000 nm) that are distributed throughout a second phase. A solution is a homogeneous mixture of two or more components. The substance that is dissolved is the solute. Updated on october 29, 2019. A) light reflects off the particles of a suspension. B) the particles of a. Suspension Colloid And Emulsion.
From www.youtube.com
SCIENCE 6 SUSPENSION AND COLLOID YouTube Suspension Colloid And Emulsion A suspension is a mixture of particles with diameters of about 1 µm (1000 nm) that are distributed throughout a second phase. The dissolving agent is the solvent. What factor distinguishes a suspension from a colloid? An emulsion is a mixture of two. Updated on october 29, 2019. Common suspensions include paint, blood, and hot. The substance that is dissolved. Suspension Colloid And Emulsion.
From www.slideserve.com
PPT Solution, Suspensions and Colloids PowerPoint Presentation, free Suspension Colloid And Emulsion A solution is a homogeneous mixture of two or more components. The substance that is dissolved is the solute. What factor distinguishes a suspension from a colloid? An emulsion, on the other hand, is a specific type of colloid where the dispersed phase consists of tiny droplets of one liquid suspended in another. The size of particles in colloids is. Suspension Colloid And Emulsion.
From www.slideserve.com
PPT Matter Properties & Change PowerPoint Presentation, free Suspension Colloid And Emulsion Common suspensions include paint, blood, and hot. The dissolving agent is the solvent. The size of particles in colloids is larger than in solution but smaller than that of suspensions. B) the particles of a suspension will. A suspension is a mixture of particles with diameters of about 1 µm (1000 nm) that are distributed throughout a second phase. In. Suspension Colloid And Emulsion.
From sciencenotes.org
What Is an Emulsion? Definition and Examples Suspension Colloid And Emulsion What factor distinguishes a suspension from a colloid? A suspension is a mixture of particles with diameters of about 1 µm (1000 nm) that are distributed throughout a second phase. Common suspensions include paint, blood, and hot. B) the particles of a suspension will. Solutions, suspensions, colloids, and other dispersions are similar but have characteristics that set each one apart. Suspension Colloid And Emulsion.
From studyzoneutsdetestable.z13.web.core.windows.net
Solutions Colloids And Suspensions Worksheet Suspension Colloid And Emulsion Updated on october 29, 2019. Solutions, suspensions, colloids, and other dispersions are similar but have characteristics that set each one apart from the others. Common suspensions include paint, blood, and hot. An emulsion is a mixture of two. The substance that is dissolved is the solute. B) the particles of a suspension will. A suspension is a mixture of particles. Suspension Colloid And Emulsion.
From www.slideserve.com
PPT Chapter 8 Solutions PowerPoint Presentation, free download ID Suspension Colloid And Emulsion Solutions, suspensions, colloids, and other dispersions are similar but have characteristics that set each one apart from the others. B) the particles of a suspension will. Updated on october 29, 2019. The dissolving agent is the solvent. The substance that is dissolved is the solute. A solution is a homogeneous mixture of two or more components. What factor distinguishes a. Suspension Colloid And Emulsion.
From pediaa.com
Difference Between Suspension and Emulsion Polymerization Definition Suspension Colloid And Emulsion Solutions, suspensions, colloids, and other dispersions are similar but have characteristics that set each one apart from the others. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a. In summary, the key difference between solution suspension and emulsion is that a solution is a mixture of two. Suspension Colloid And Emulsion.
From pt.slideshare.net
Solutions, suspensions, and colloids Suspension Colloid And Emulsion The substance that is dissolved is the solute. What factor distinguishes a suspension from a colloid? The size of particles in colloids is larger than in solution but smaller than that of suspensions. Solutions, suspensions, colloids, and other dispersions are similar but have characteristics that set each one apart from the others. B) the particles of a suspension will. Updated. Suspension Colloid And Emulsion.
From plantlet.org
Mixture Types Solution, Suspension, Colloids & Others Plantlet Suspension Colloid And Emulsion An emulsion, on the other hand, is a specific type of colloid where the dispersed phase consists of tiny droplets of one liquid suspended in another. The dissolving agent is the solvent. The size of particles in colloids is larger than in solution but smaller than that of suspensions. Common suspensions include paint, blood, and hot. The substance that is. Suspension Colloid And Emulsion.
From www.slideserve.com
PPT Water PowerPoint Presentation, free download ID2182578 Suspension Colloid And Emulsion A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a. A) light reflects off the particles of a suspension. B) the particles of a suspension will. A solution is a homogeneous mixture of two or more components. Updated on october 29, 2019. An emulsion is a mixture of. Suspension Colloid And Emulsion.
From www.vecteezy.com
True Solution, Colloid solution and Suspension three different types of Suspension Colloid And Emulsion What factor distinguishes a suspension from a colloid? The substance that is dissolved is the solute. B) the particles of a suspension will. Common suspensions include paint, blood, and hot. A solution is a homogeneous mixture of two or more components. The dissolving agent is the solvent. Solutions, suspensions, colloids, and other dispersions are similar but have characteristics that set. Suspension Colloid And Emulsion.
From www.youtube.com
Colloids, Solutions & Suspensions YouTube Suspension Colloid And Emulsion A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a. A solution is a homogeneous mixture of two or more components. Updated on october 29, 2019. An emulsion, on the other hand, is a specific type of colloid where the dispersed phase consists of tiny droplets of one. Suspension Colloid And Emulsion.
From www.youtube.com
Solution Suspension Colloid YouTube Suspension Colloid And Emulsion A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a. Common suspensions include paint, blood, and hot. An emulsion, on the other hand, is a specific type of colloid where the dispersed phase consists of tiny droplets of one liquid suspended in another. Solutions, suspensions, colloids, and other. Suspension Colloid And Emulsion.
From www.slideserve.com
PPT 2 PowerPoint Presentation, free download ID6415741 Suspension Colloid And Emulsion A) light reflects off the particles of a suspension. An emulsion is a mixture of two. In summary, the key difference between solution suspension and emulsion is that a solution is a mixture of two and suspension is a heterogeneous. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution. Suspension Colloid And Emulsion.
From quizizz.com
Describe the appearance of Colloids and Suspension. Quizizz Suspension Colloid And Emulsion A solution is a homogeneous mixture of two or more components. A) light reflects off the particles of a suspension. The size of particles in colloids is larger than in solution but smaller than that of suspensions. The substance that is dissolved is the solute. An emulsion, on the other hand, is a specific type of colloid where the dispersed. Suspension Colloid And Emulsion.
From www.slideserve.com
PPT Chapter 12 SOLUTIONS PowerPoint Presentation, free download ID Suspension Colloid And Emulsion A solution is a homogeneous mixture of two or more components. An emulsion is a mixture of two. Updated on october 29, 2019. The size of particles in colloids is larger than in solution but smaller than that of suspensions. The dissolving agent is the solvent. Common suspensions include paint, blood, and hot. B) the particles of a suspension will.. Suspension Colloid And Emulsion.
From www.stepbystep.com
Difference Between Suspension and Colloid Suspension Colloid And Emulsion The substance that is dissolved is the solute. A) light reflects off the particles of a suspension. Common suspensions include paint, blood, and hot. A suspension is a mixture of particles with diameters of about 1 µm (1000 nm) that are distributed throughout a second phase. What factor distinguishes a suspension from a colloid? Updated on october 29, 2019. The. Suspension Colloid And Emulsion.
From www.youtube.com
Chemistry 9.4 Solutions, Colloids and Suspensions YouTube Suspension Colloid And Emulsion The size of particles in colloids is larger than in solution but smaller than that of suspensions. An emulsion is a mixture of two. The substance that is dissolved is the solute. A) light reflects off the particles of a suspension. A suspension is a mixture of particles with diameters of about 1 µm (1000 nm) that are distributed throughout. Suspension Colloid And Emulsion.