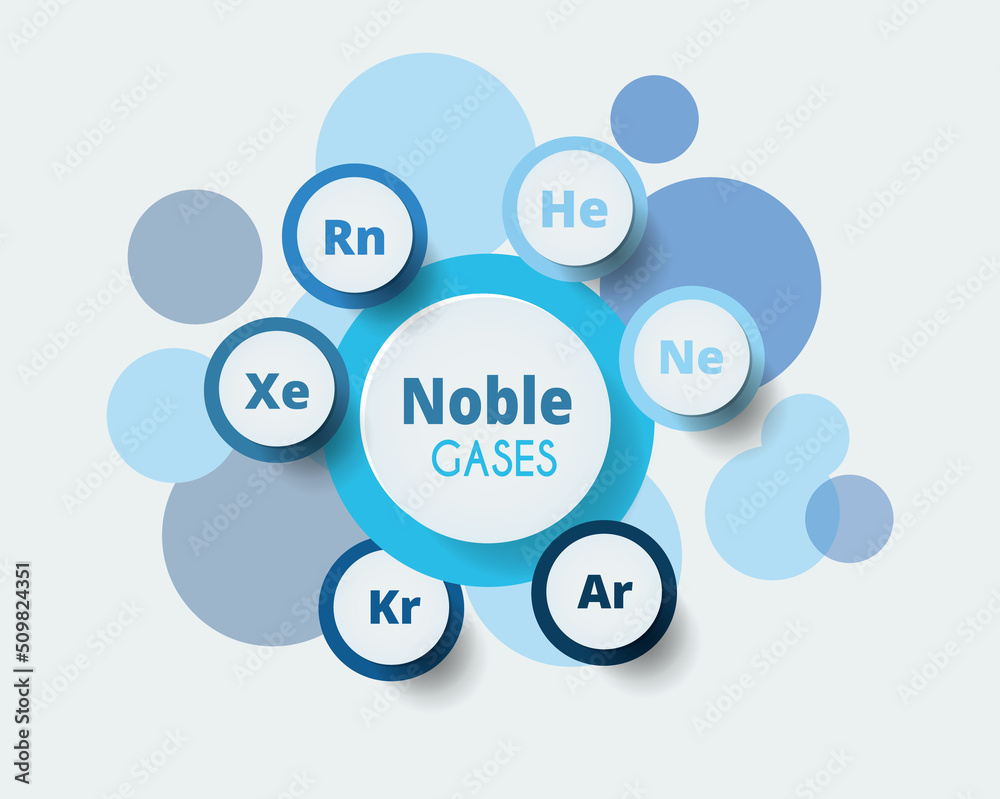
Argon Krypton Xenon Radon . The elements in group 18 are the noble gases (helium, neon, argon, krypton, xenon, and radon). Helium, neon, argon, krypton, xenon, radon, and oganesson. They are nearly inert because the atoms have a full valence electron shell, with little tendency to accept or donate electrons to form chemical bonds. Then, in a single year (1898), he discovered the next three noble gases: Krypton (kr), from the greek kryptos, meaning “hidden,” was identified. The reactivity of radon means that it can be chemically scrubbed from the air in uranium mines and other mines. The noble gases make up the last column of elements in the periodic table. They earned the name “noble” because they. They are commonly called group 18, the inert gases, the rare gases, the helium family, or the. There are seven noble gas elements: Noble gases are the least reactive chemical elements. Excimer lasers use compounds of argon, krypton.
from stock.adobe.com
They earned the name “noble” because they. The noble gases make up the last column of elements in the periodic table. Helium, neon, argon, krypton, xenon, radon, and oganesson. The elements in group 18 are the noble gases (helium, neon, argon, krypton, xenon, and radon). There are seven noble gas elements: They are nearly inert because the atoms have a full valence electron shell, with little tendency to accept or donate electrons to form chemical bonds. Krypton (kr), from the greek kryptos, meaning “hidden,” was identified. They are commonly called group 18, the inert gases, the rare gases, the helium family, or the. Excimer lasers use compounds of argon, krypton. The reactivity of radon means that it can be chemically scrubbed from the air in uranium mines and other mines.
Noble gases. Vector illustration. Periodic table of elements. Chemical
Argon Krypton Xenon Radon Then, in a single year (1898), he discovered the next three noble gases: Noble gases are the least reactive chemical elements. The reactivity of radon means that it can be chemically scrubbed from the air in uranium mines and other mines. The elements in group 18 are the noble gases (helium, neon, argon, krypton, xenon, and radon). Then, in a single year (1898), he discovered the next three noble gases: They earned the name “noble” because they. Excimer lasers use compounds of argon, krypton. Helium, neon, argon, krypton, xenon, radon, and oganesson. Krypton (kr), from the greek kryptos, meaning “hidden,” was identified. They are nearly inert because the atoms have a full valence electron shell, with little tendency to accept or donate electrons to form chemical bonds. They are commonly called group 18, the inert gases, the rare gases, the helium family, or the. The noble gases make up the last column of elements in the periodic table. There are seven noble gas elements:
From de.academic.ru
Edelgase Argon Krypton Xenon Radon The reactivity of radon means that it can be chemically scrubbed from the air in uranium mines and other mines. Helium, neon, argon, krypton, xenon, radon, and oganesson. They are nearly inert because the atoms have a full valence electron shell, with little tendency to accept or donate electrons to form chemical bonds. There are seven noble gas elements: The. Argon Krypton Xenon Radon.
From spanish.alibaba.com
Juego De 8 Tipos De Gases Noble Sellados En Ampollas Helio Neón Argón Argon Krypton Xenon Radon They are nearly inert because the atoms have a full valence electron shell, with little tendency to accept or donate electrons to form chemical bonds. They earned the name “noble” because they. Noble gases are the least reactive chemical elements. The elements in group 18 are the noble gases (helium, neon, argon, krypton, xenon, and radon). Helium, neon, argon, krypton,. Argon Krypton Xenon Radon.
From www.examples.com
Radon (Rn) Definition, Preparation, Properties, Uses, Compounds Argon Krypton Xenon Radon Krypton (kr), from the greek kryptos, meaning “hidden,” was identified. They are commonly called group 18, the inert gases, the rare gases, the helium family, or the. There are seven noble gas elements: The elements in group 18 are the noble gases (helium, neon, argon, krypton, xenon, and radon). The noble gases make up the last column of elements in. Argon Krypton Xenon Radon.
From ubicaciondepersonas.cdmx.gob.mx
Argon Lights ubicaciondepersonas.cdmx.gob.mx Argon Krypton Xenon Radon They are nearly inert because the atoms have a full valence electron shell, with little tendency to accept or donate electrons to form chemical bonds. There are seven noble gas elements: The elements in group 18 are the noble gases (helium, neon, argon, krypton, xenon, and radon). The reactivity of radon means that it can be chemically scrubbed from the. Argon Krypton Xenon Radon.
From www.examples.com
What is Radon(Rn)? Preparation, Properties, Uses, Compounds, Reactivity Argon Krypton Xenon Radon The elements in group 18 are the noble gases (helium, neon, argon, krypton, xenon, and radon). The reactivity of radon means that it can be chemically scrubbed from the air in uranium mines and other mines. The noble gases make up the last column of elements in the periodic table. Then, in a single year (1898), he discovered the next. Argon Krypton Xenon Radon.
From www.researchgate.net
(PDF) Neón, argón, kriptón, xenón y radón Argon Krypton Xenon Radon Krypton (kr), from the greek kryptos, meaning “hidden,” was identified. Helium, neon, argon, krypton, xenon, radon, and oganesson. The noble gases make up the last column of elements in the periodic table. The reactivity of radon means that it can be chemically scrubbed from the air in uranium mines and other mines. They earned the name “noble” because they. Noble. Argon Krypton Xenon Radon.
From material-properties.org
Helium and Argon Comparison Properties Material Properties Argon Krypton Xenon Radon Krypton (kr), from the greek kryptos, meaning “hidden,” was identified. There are seven noble gas elements: Helium, neon, argon, krypton, xenon, radon, and oganesson. They are nearly inert because the atoms have a full valence electron shell, with little tendency to accept or donate electrons to form chemical bonds. Then, in a single year (1898), he discovered the next three. Argon Krypton Xenon Radon.
From blog.knitwithattitude.com
A Year Long KnitALong Sockitude Kits The Knit with attitude Blog Argon Krypton Xenon Radon They are nearly inert because the atoms have a full valence electron shell, with little tendency to accept or donate electrons to form chemical bonds. The noble gases make up the last column of elements in the periodic table. They are commonly called group 18, the inert gases, the rare gases, the helium family, or the. There are seven noble. Argon Krypton Xenon Radon.
From stock.adobe.com
Noble gases. Vector illustration. Periodic table of elements. Chemical Argon Krypton Xenon Radon Noble gases are the least reactive chemical elements. They are nearly inert because the atoms have a full valence electron shell, with little tendency to accept or donate electrons to form chemical bonds. Excimer lasers use compounds of argon, krypton. The reactivity of radon means that it can be chemically scrubbed from the air in uranium mines and other mines.. Argon Krypton Xenon Radon.
From www.schubu.at
Chemische Bindungen Chemie SchuBu Argon Krypton Xenon Radon The elements in group 18 are the noble gases (helium, neon, argon, krypton, xenon, and radon). Helium, neon, argon, krypton, xenon, radon, and oganesson. There are seven noble gas elements: They are commonly called group 18, the inert gases, the rare gases, the helium family, or the. Then, in a single year (1898), he discovered the next three noble gases:. Argon Krypton Xenon Radon.
From www.slideserve.com
PPT The halogens are in group 7 PowerPoint Presentation, free Argon Krypton Xenon Radon Excimer lasers use compounds of argon, krypton. The elements in group 18 are the noble gases (helium, neon, argon, krypton, xenon, and radon). The noble gases make up the last column of elements in the periodic table. The reactivity of radon means that it can be chemically scrubbed from the air in uranium mines and other mines. They earned the. Argon Krypton Xenon Radon.
From archiv.akademon.cz
Akademon Argon Krypton Xenon Radon Helium, neon, argon, krypton, xenon, radon, and oganesson. There are seven noble gas elements: The noble gases make up the last column of elements in the periodic table. Noble gases are the least reactive chemical elements. The reactivity of radon means that it can be chemically scrubbed from the air in uranium mines and other mines. Excimer lasers use compounds. Argon Krypton Xenon Radon.
From saintwah.en.made-in-china.com
Industrial Gas Storage Helium, Neon, Argon, Krypton, Xenon, Radon Argon Krypton Xenon Radon Helium, neon, argon, krypton, xenon, radon, and oganesson. They earned the name “noble” because they. Noble gases are the least reactive chemical elements. Excimer lasers use compounds of argon, krypton. They are commonly called group 18, the inert gases, the rare gases, the helium family, or the. The noble gases make up the last column of elements in the periodic. Argon Krypton Xenon Radon.
From turnerperiodictable.weebly.com
Group 18 Classification of Elements Argon Krypton Xenon Radon They are commonly called group 18, the inert gases, the rare gases, the helium family, or the. Helium, neon, argon, krypton, xenon, radon, and oganesson. The noble gases make up the last column of elements in the periodic table. They are nearly inert because the atoms have a full valence electron shell, with little tendency to accept or donate electrons. Argon Krypton Xenon Radon.
From www.scribd.com
People Also Ask Helium (He), Neon (Ne), Argon (Ar), Krypton (KR Argon Krypton Xenon Radon The noble gases make up the last column of elements in the periodic table. Then, in a single year (1898), he discovered the next three noble gases: Excimer lasers use compounds of argon, krypton. The reactivity of radon means that it can be chemically scrubbed from the air in uranium mines and other mines. They are nearly inert because the. Argon Krypton Xenon Radon.
From www.ebay.com
Set Noble gases(inert gases/inactive gases)TUBEsHelium Neon Argon Argon Krypton Xenon Radon The reactivity of radon means that it can be chemically scrubbed from the air in uranium mines and other mines. Helium, neon, argon, krypton, xenon, radon, and oganesson. Krypton (kr), from the greek kryptos, meaning “hidden,” was identified. They are nearly inert because the atoms have a full valence electron shell, with little tendency to accept or donate electrons to. Argon Krypton Xenon Radon.
From de.academic.ru
Edelgase Argon Krypton Xenon Radon Krypton (kr), from the greek kryptos, meaning “hidden,” was identified. They are commonly called group 18, the inert gases, the rare gases, the helium family, or the. Helium, neon, argon, krypton, xenon, radon, and oganesson. Then, in a single year (1898), he discovered the next three noble gases: Noble gases are the least reactive chemical elements. The elements in group. Argon Krypton Xenon Radon.
From stock.adobe.com
Noble gases. Vector 3d illustration. Helium, neon, argon, krypton Argon Krypton Xenon Radon Excimer lasers use compounds of argon, krypton. The elements in group 18 are the noble gases (helium, neon, argon, krypton, xenon, and radon). They are commonly called group 18, the inert gases, the rare gases, the helium family, or the. They are nearly inert because the atoms have a full valence electron shell, with little tendency to accept or donate. Argon Krypton Xenon Radon.
From www.slideserve.com
PPT METALS, NONMETALS, METALLOIDS, & NOBLE GASES PowerPoint Argon Krypton Xenon Radon There are seven noble gas elements: They earned the name “noble” because they. The noble gases make up the last column of elements in the periodic table. Krypton (kr), from the greek kryptos, meaning “hidden,” was identified. The reactivity of radon means that it can be chemically scrubbed from the air in uranium mines and other mines. They are nearly. Argon Krypton Xenon Radon.
From www.reddit.com
Why do those noble gasses have electronegativity? (Krypton, Xenon, and Argon Krypton Xenon Radon Then, in a single year (1898), he discovered the next three noble gases: They are commonly called group 18, the inert gases, the rare gases, the helium family, or the. Krypton (kr), from the greek kryptos, meaning “hidden,” was identified. Noble gases are the least reactive chemical elements. They earned the name “noble” because they. The elements in group 18. Argon Krypton Xenon Radon.
From www.walmart.com
The Noble Gases Helium, Neon, Argon, Krypton, Xenon, Radon Walmart Argon Krypton Xenon Radon The elements in group 18 are the noble gases (helium, neon, argon, krypton, xenon, and radon). Krypton (kr), from the greek kryptos, meaning “hidden,” was identified. They are nearly inert because the atoms have a full valence electron shell, with little tendency to accept or donate electrons to form chemical bonds. There are seven noble gas elements: They are commonly. Argon Krypton Xenon Radon.
From docslib.org
The Noble Gases Helium, Neon, Argon, Krypton, Xenon, and Radon DocsLib Argon Krypton Xenon Radon They are commonly called group 18, the inert gases, the rare gases, the helium family, or the. The elements in group 18 are the noble gases (helium, neon, argon, krypton, xenon, and radon). Krypton (kr), from the greek kryptos, meaning “hidden,” was identified. Excimer lasers use compounds of argon, krypton. The noble gases make up the last column of elements. Argon Krypton Xenon Radon.
From www.haikudeck.com
Noble Gases/Argon by nathan.sikkink Argon Krypton Xenon Radon The noble gases make up the last column of elements in the periodic table. Helium, neon, argon, krypton, xenon, radon, and oganesson. Krypton (kr), from the greek kryptos, meaning “hidden,” was identified. The elements in group 18 are the noble gases (helium, neon, argon, krypton, xenon, and radon). There are seven noble gas elements: Excimer lasers use compounds of argon,. Argon Krypton Xenon Radon.
From www.youtube.com
Group 8 Elements. Noble Gases. Helium, Neon, Argon, Krypton, Xenon Argon Krypton Xenon Radon They earned the name “noble” because they. The noble gases make up the last column of elements in the periodic table. Noble gases are the least reactive chemical elements. Then, in a single year (1898), he discovered the next three noble gases: Krypton (kr), from the greek kryptos, meaning “hidden,” was identified. They are nearly inert because the atoms have. Argon Krypton Xenon Radon.
From www.researchgate.net
Same as in figure 1 except for argon, krypton and xenon. Symbols used Argon Krypton Xenon Radon They earned the name “noble” because they. Noble gases are the least reactive chemical elements. Excimer lasers use compounds of argon, krypton. They are commonly called group 18, the inert gases, the rare gases, the helium family, or the. The elements in group 18 are the noble gases (helium, neon, argon, krypton, xenon, and radon). There are seven noble gas. Argon Krypton Xenon Radon.
From stock.adobe.com
Noble gases. Helium balloons. Helium, argon, neon, krypton, xenon Argon Krypton Xenon Radon The elements in group 18 are the noble gases (helium, neon, argon, krypton, xenon, and radon). They earned the name “noble” because they. Noble gases are the least reactive chemical elements. The noble gases make up the last column of elements in the periodic table. They are commonly called group 18, the inert gases, the rare gases, the helium family,. Argon Krypton Xenon Radon.
From avopix.com
Noble gases. Vector illustration. Periodic table Royalty Free Stock Argon Krypton Xenon Radon They earned the name “noble” because they. They are nearly inert because the atoms have a full valence electron shell, with little tendency to accept or donate electrons to form chemical bonds. They are commonly called group 18, the inert gases, the rare gases, the helium family, or the. Krypton (kr), from the greek kryptos, meaning “hidden,” was identified. The. Argon Krypton Xenon Radon.
From www.atmo.arizona.edu
Lecture 1 Composition of the atmosphere Argon Krypton Xenon Radon Krypton (kr), from the greek kryptos, meaning “hidden,” was identified. There are seven noble gas elements: Then, in a single year (1898), he discovered the next three noble gases: Noble gases are the least reactive chemical elements. They are commonly called group 18, the inert gases, the rare gases, the helium family, or the. Excimer lasers use compounds of argon,. Argon Krypton Xenon Radon.
From www.youtube.com
The Noble "Gas" Family Helium, Neon, Argon, Krypton, Xenon, Radon Argon Krypton Xenon Radon They are commonly called group 18, the inert gases, the rare gases, the helium family, or the. Helium, neon, argon, krypton, xenon, radon, and oganesson. The noble gases make up the last column of elements in the periodic table. Excimer lasers use compounds of argon, krypton. The elements in group 18 are the noble gases (helium, neon, argon, krypton, xenon,. Argon Krypton Xenon Radon.
From www.atmo.arizona.edu
Lecture 1 Composition of the atmosphere Argon Krypton Xenon Radon The reactivity of radon means that it can be chemically scrubbed from the air in uranium mines and other mines. Krypton (kr), from the greek kryptos, meaning “hidden,” was identified. Excimer lasers use compounds of argon, krypton. They are nearly inert because the atoms have a full valence electron shell, with little tendency to accept or donate electrons to form. Argon Krypton Xenon Radon.
From www.slideserve.com
PPT Hosted by Mr. Brown PowerPoint Presentation, free download ID Argon Krypton Xenon Radon The elements in group 18 are the noble gases (helium, neon, argon, krypton, xenon, and radon). Noble gases are the least reactive chemical elements. Krypton (kr), from the greek kryptos, meaning “hidden,” was identified. They are nearly inert because the atoms have a full valence electron shell, with little tendency to accept or donate electrons to form chemical bonds. Excimer. Argon Krypton Xenon Radon.
From www.reddit.com
There’s antimony, arsenic, aluminum, selenium, And hydrogen and oxygen Argon Krypton Xenon Radon They are commonly called group 18, the inert gases, the rare gases, the helium family, or the. Noble gases are the least reactive chemical elements. Krypton (kr), from the greek kryptos, meaning “hidden,” was identified. They are nearly inert because the atoms have a full valence electron shell, with little tendency to accept or donate electrons to form chemical bonds.. Argon Krypton Xenon Radon.
From argonnekonba.blogspot.com
Argon Argon Xenon Krypton Argon Krypton Xenon Radon Then, in a single year (1898), he discovered the next three noble gases: Noble gases are the least reactive chemical elements. They earned the name “noble” because they. The reactivity of radon means that it can be chemically scrubbed from the air in uranium mines and other mines. Excimer lasers use compounds of argon, krypton. Krypton (kr), from the greek. Argon Krypton Xenon Radon.
From www.revistasusana.com
Unveiling the Mystery The Sixth Noble Gas and the Big Bang Theory Argon Krypton Xenon Radon Then, in a single year (1898), he discovered the next three noble gases: They earned the name “noble” because they. The reactivity of radon means that it can be chemically scrubbed from the air in uranium mines and other mines. They are nearly inert because the atoms have a full valence electron shell, with little tendency to accept or donate. Argon Krypton Xenon Radon.
From www.raremetalbuy.com
Rare gas Hydrogen nitrogen and oxygen helium neon argon krypton xenon Argon Krypton Xenon Radon The reactivity of radon means that it can be chemically scrubbed from the air in uranium mines and other mines. Then, in a single year (1898), he discovered the next three noble gases: Noble gases are the least reactive chemical elements. Excimer lasers use compounds of argon, krypton. Krypton (kr), from the greek kryptos, meaning “hidden,” was identified. They are. Argon Krypton Xenon Radon.