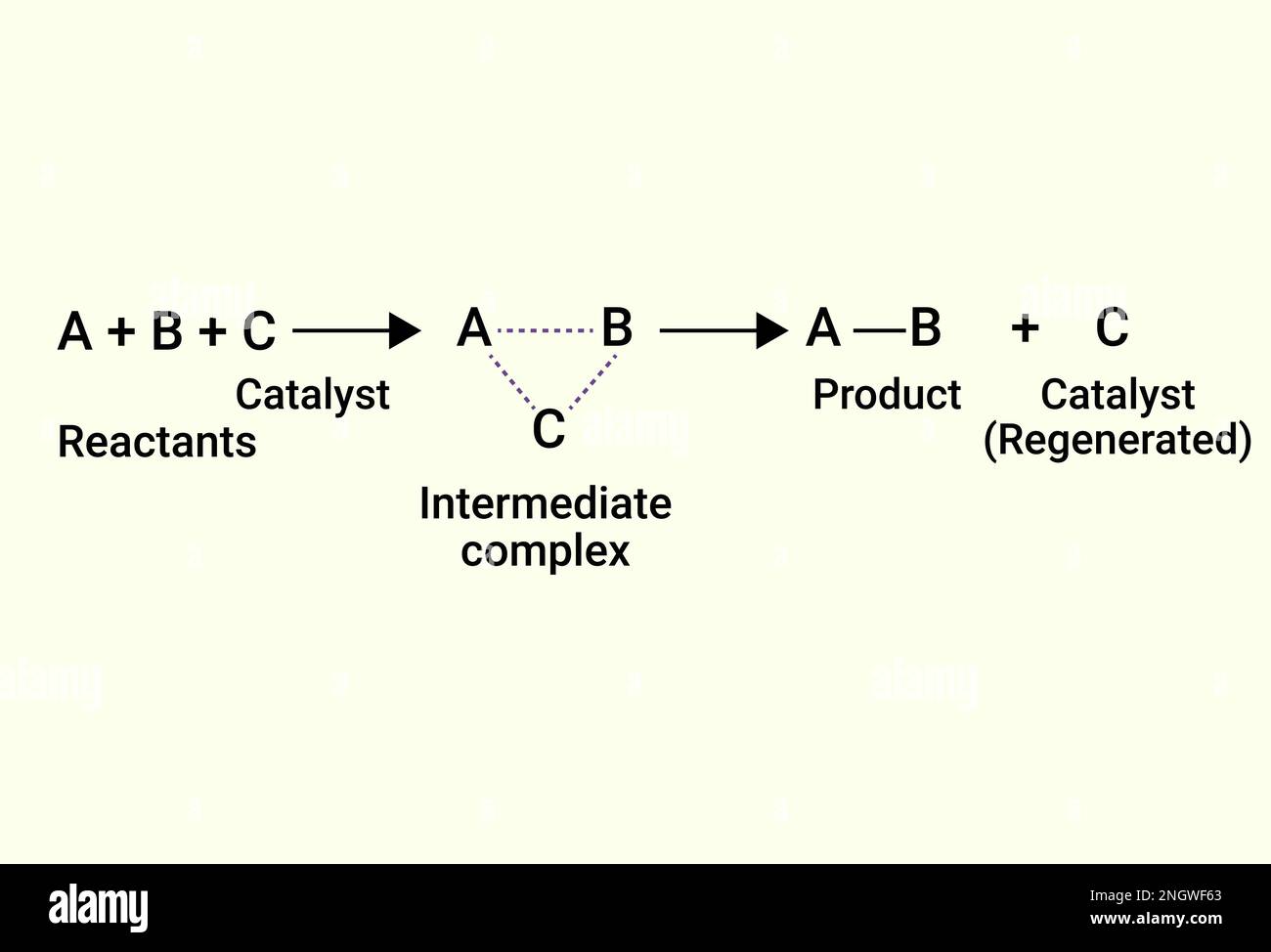
Catalyst Reaction Is . A molecule may have certain properties that let it lure away atoms from another molecule. in chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. This is like swapping partners at a square dance. catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. The atoms also make new bonds with different atoms. List examples of catalysis in. Sometimes, those partnerships are easy to break. explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams; during any chemical reaction, molecules break chemical bonds between their atoms. A catalyst is a chemical.
from www.alamy.com
List examples of catalysis in. catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. This is like swapping partners at a square dance. A catalyst is a chemical. A molecule may have certain properties that let it lure away atoms from another molecule. In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. in chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams;
Chemical Reactions of catalyst and product Stock Vector Image & Art Alamy
Catalyst Reaction Is explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams; List examples of catalysis in. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. Sometimes, those partnerships are easy to break. during any chemical reaction, molecules break chemical bonds between their atoms. A molecule may have certain properties that let it lure away atoms from another molecule. The atoms also make new bonds with different atoms. explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams; catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. This is like swapping partners at a square dance. A catalyst is a chemical. in chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it.
From www.slideserve.com
PPT CATALYSIS AND CATALYTIC REACTION MECHANISM PART 1 PowerPoint Presentation ID2087208 Catalyst Reaction Is in chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. This is like swapping partners at a square dance. The atoms also make new bonds with different atoms. Sometimes, those partnerships are easy to break. catalysts allow a reaction to proceed via a pathway that has. Catalyst Reaction Is.
From 2012books.lardbucket.org
Catalysis Catalyst Reaction Is catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. The atoms also make new bonds with different atoms. List examples of catalysis in. during any chemical reaction, molecules break chemical bonds between their atoms. This is like swapping partners at a square dance. A molecule may have certain properties. Catalyst Reaction Is.
From nonsibihighschool.org
Section 184 Catalysis Catalyst Reaction Is catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. The atoms also make new bonds with different atoms. Sometimes, those partnerships are easy to break. List examples of catalysis in. in chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being. Catalyst Reaction Is.
From www.slideshare.net
Biology 2.4 Catalyst Reaction Is Sometimes, those partnerships are easy to break. The atoms also make new bonds with different atoms. explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams; in chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. List examples of catalysis in.. Catalyst Reaction Is.
From www.researchgate.net
Catalytic processes on a solid catalyst. Download Scientific Diagram Catalyst Reaction Is during any chemical reaction, molecules break chemical bonds between their atoms. explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams; catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. catalysts allow a reaction to proceed via a pathway that has a. Catalyst Reaction Is.
From www.youtube.com
Catalysts Chemistry Khan Academy YouTube Catalyst Reaction Is catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. in chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. This is like swapping partners at a square dance. In heterogeneous catalysis, catalysts provide a surface to which. Catalyst Reaction Is.
From www.youtube.com
How To Identify The Intermediate & Catalyst In a Reaction Mechanism Chemistry YouTube Catalyst Reaction Is during any chemical reaction, molecules break chemical bonds between their atoms. A catalyst is a chemical. Sometimes, those partnerships are easy to break. This is like swapping partners at a square dance. catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. List examples of catalysis in. explain the. Catalyst Reaction Is.
From www.dreamstime.com
Enzyme As Catalyst in Chemical Reactions Stock Vector Illustration of broken, diagram 215849462 Catalyst Reaction Is In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. This is like swapping partners at a square dance. catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams;. Catalyst Reaction Is.
From www.alamy.com
Chemical Reactions of catalyst and product Stock Vector Image & Art Alamy Catalyst Reaction Is explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams; in chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. In heterogeneous catalysis, catalysts. Catalyst Reaction Is.
From www.youtube.com
Identifying catalysts in a reaction YouTube Catalyst Reaction Is catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. List examples of catalysis in. in chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a. Catalyst Reaction Is.
From jackwestin.com
Rate Processes Catalysts Rate Processes In Chemical Reactions And Equilibrium MCAT Catalyst Reaction Is catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. A molecule may have certain properties that let it lure away atoms from another molecule. In heterogeneous catalysis, catalysts provide a surface to which. Catalyst Reaction Is.
From fyochldly.blob.core.windows.net
Catalyst Chemical Equation Reaction at Justin Samuels blog Catalyst Reaction Is catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. in chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed. Catalyst Reaction Is.
From www.catalystseurope.org
What are catalysts? Catalyst Reaction Is catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. in chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. during any chemical reaction, molecules break chemical bonds between their atoms. explain the function of. Catalyst Reaction Is.
From www.chemistrystudent.com
Heterogeneous Catalysis (ALevel) ChemistryStudent Catalyst Reaction Is In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. during any chemical reaction, molecules break chemical bonds between their atoms. in chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. catalysts allow a reaction to proceed via a. Catalyst Reaction Is.
From www.researchgate.net
1 Schematic illustration of a catalytic process showing "A" and "B"... Download Scientific Diagram Catalyst Reaction Is in chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams; List examples of catalysis in. catalysis is the process that alters the rate of a chemical reaction under the influence. Catalyst Reaction Is.
From scitechdaily.com
Science Made Simple What Are Catalysts? Catalyst Reaction Is explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams; List examples of catalysis in. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. The atoms also make new bonds with different atoms. A molecule may have certain properties that let it lure. Catalyst Reaction Is.
From large.stanford.edu
Catalysts in 21st Century Energy Catalyst Reaction Is catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. A molecule may have certain properties that let it lure away atoms from another molecule. A catalyst is a chemical. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. in chemistry. Catalyst Reaction Is.
From fyorswrmb.blob.core.windows.net
How Does Catalyst Affect The Rate Of Chemical Reaction at Jermaine Carroll blog Catalyst Reaction Is A catalyst is a chemical. In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams; catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. This is like. Catalyst Reaction Is.
From www.researchgate.net
Reaction coordinate diagram showing the working principle of a catalyst. Download Scientific Catalyst Reaction Is In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. The atoms also make new bonds with different atoms. List examples of catalysis in. catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. This is like swapping partners at a square dance. catalysts. Catalyst Reaction Is.
From www.dreamstime.com
Catalyst Surface with Catalytic Reaction Stock Vector Illustration of bind, matter 218305638 Catalyst Reaction Is catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. during any chemical reaction, molecules break chemical bonds between their atoms. The atoms also make new bonds with different atoms. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Sometimes, those. Catalyst Reaction Is.
From www.slideserve.com
PPT Chapter 11 Chemical Reactions PowerPoint Presentation, free download ID6634764 Catalyst Reaction Is catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. A molecule may have certain properties that let it lure away atoms from another molecule. Sometimes, those partnerships are easy to break. during. Catalyst Reaction Is.
From www.youtube.com
What Are Catalysts? Reactions Chemistry FuseSchool YouTube Catalyst Reaction Is A catalyst is a chemical. in chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. catalyst, in chemistry, any substance that increases the rate of a reaction. Catalyst Reaction Is.
From www.mometrix.com
What is a Catalyst? Chemistry Review (Video) Catalyst Reaction Is A catalyst is a chemical. The atoms also make new bonds with different atoms. This is like swapping partners at a square dance. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. in chemistry and biology, a catalyst is a substance the increases the rate of a chemical. Catalyst Reaction Is.
From www.slideserve.com
PPT CATALYSIS AND CATALYTIC REACTION MECHANISM PART 1 PowerPoint Presentation ID2087208 Catalyst Reaction Is catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. List examples of catalysis in. during any chemical reaction, molecules break chemical bonds between their atoms. In heterogeneous catalysis, catalysts. Catalyst Reaction Is.
From www.labunlimited.com
Solid Phase Catalysis in Continuous Flow Chemistry Lab Unlimited Catalyst Reaction Is in chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams; catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. catalyst,. Catalyst Reaction Is.
From www.slideserve.com
PPT Reaction Mechanism PowerPoint Presentation, free download ID2230405 Catalyst Reaction Is catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. A catalyst is a chemical. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams;. Catalyst Reaction Is.
From diagramlisthavens.z21.web.core.windows.net
Reaction Coordinate Diagram With Catalyst Catalyst Reaction Is catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. in chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. List. Catalyst Reaction Is.
From www.pinterest.com
Catalyst Easy Science Energy activities, Chemical reactions, Chemistry education Catalyst Reaction Is A molecule may have certain properties that let it lure away atoms from another molecule. A catalyst is a chemical. In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. This is like swapping partners at a. Catalyst Reaction Is.
From hxegwtrde.blob.core.windows.net
What Is The Catalyst In A Chemical Reaction at Keith Bridges blog Catalyst Reaction Is A catalyst is a chemical. A molecule may have certain properties that let it lure away atoms from another molecule. during any chemical reaction, molecules break chemical bonds between their atoms. The atoms also make new bonds with different atoms. In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. This is like. Catalyst Reaction Is.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis Catalyst Reaction Is in chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. A catalyst is a chemical. The atoms also make new bonds with different atoms. A molecule may have certain properties that let it lure away atoms from another molecule. catalysis is the process that alters the. Catalyst Reaction Is.
From www.pinterest.com
Catalyst speeds up a chemical reaction by lowering the activation energy Ea of the overall Catalyst Reaction Is Sometimes, those partnerships are easy to break. The atoms also make new bonds with different atoms. A molecule may have certain properties that let it lure away atoms from another molecule. A catalyst is a chemical. In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. in chemistry and biology, a catalyst is. Catalyst Reaction Is.
From fyolrjkxj.blob.core.windows.net
How A Catalyst Speeds Up A Reaction at Theodora Sanchez blog Catalyst Reaction Is explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams; in chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. A molecule may have certain properties that let it lure away atoms from another molecule. Sometimes, those partnerships are easy to. Catalyst Reaction Is.
From www.britannica.com
Catalyst Examples, Definition, & Facts Britannica Catalyst Reaction Is catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. This is like swapping partners at a square dance. during any chemical reaction, molecules break chemical bonds between their atoms. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Sometimes, those partnerships. Catalyst Reaction Is.
From www.slideshare.net
Catalyst Catalyst Reaction Is In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. during any chemical reaction, molecules break chemical bonds between their atoms. A molecule may have certain properties that let it lure away atoms from another molecule. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than. Catalyst Reaction Is.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Catalyst Reaction Is explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams; List examples of catalysis in. Sometimes, those partnerships are easy to break. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. The atoms also make new bonds with different atoms. In heterogeneous catalysis, catalysts provide a. Catalyst Reaction Is.