How Does An Alkaline Buffer Work . An alkaline buffer solution has a ph greater than 7. Alkaline buffer solutions are commonly made from a weak base and one of its salts. Learn about acidic and alkaline buffers, their preparation, mechanism, and applications with examples and problems. Alkaline buffer solutions are commonly made. Learn how buffers protect against ph changes when strong acid or base is added. This tutorial explains the concept of buffer capacity and how to calculate it, with examples and diagrams. An alkaline buffer solution has a ph greater than 7. It is able to neutralize small amounts of added acid or base, thus. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components.
from kyvokedymolyb.modellervefiyatlar.com
An alkaline buffer solution has a ph greater than 7. This tutorial explains the concept of buffer capacity and how to calculate it, with examples and diagrams. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Learn how buffers protect against ph changes when strong acid or base is added. An alkaline buffer solution has a ph greater than 7. Learn about acidic and alkaline buffers, their preparation, mechanism, and applications with examples and problems. Alkaline buffer solutions are commonly made. It is able to neutralize small amounts of added acid or base, thus. Alkaline buffer solutions are commonly made from a weak base and one of its salts.
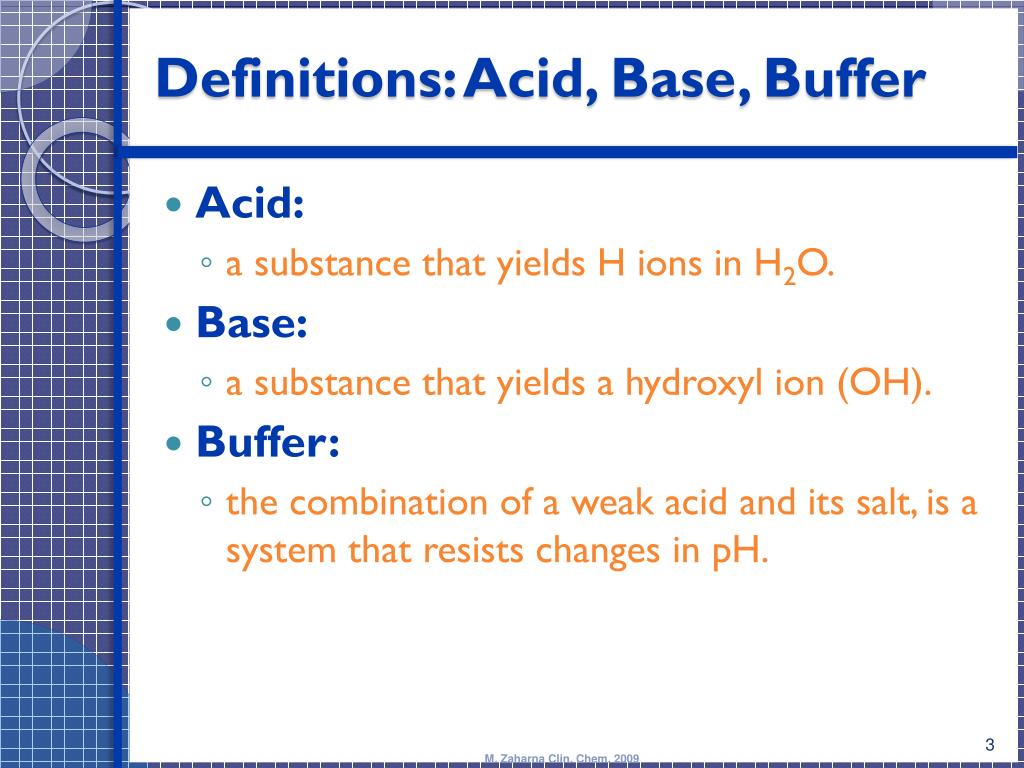
Acid Base Buffers
How Does An Alkaline Buffer Work It is able to neutralize small amounts of added acid or base, thus. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Alkaline buffer solutions are commonly made. Learn how buffers protect against ph changes when strong acid or base is added. An alkaline buffer solution has a ph greater than 7. Learn about acidic and alkaline buffers, their preparation, mechanism, and applications with examples and problems. Alkaline buffer solutions are commonly made from a weak base and one of its salts. This tutorial explains the concept of buffer capacity and how to calculate it, with examples and diagrams. It is able to neutralize small amounts of added acid or base, thus. An alkaline buffer solution has a ph greater than 7.
From www.vrogue.co
How Buffers Work vrogue.co How Does An Alkaline Buffer Work It is able to neutralize small amounts of added acid or base, thus. An alkaline buffer solution has a ph greater than 7. Learn how buffers protect against ph changes when strong acid or base is added. An alkaline buffer solution has a ph greater than 7. This tutorial explains the concept of buffer capacity and how to calculate it,. How Does An Alkaline Buffer Work.
From www.meritnation.com
How does an alkaline cell work Science Electricity 10526719 How Does An Alkaline Buffer Work An alkaline buffer solution has a ph greater than 7. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Alkaline buffer solutions are commonly made from a weak base and one of its salts. It is able to neutralize small amounts of added acid or base, thus. Alkaline buffer solutions. How Does An Alkaline Buffer Work.
From atelier-yuwa.ciao.jp
A Schematic Illustration Of A Basic Alkaline Water Electrolysis How Does An Alkaline Buffer Work Alkaline buffer solutions are commonly made. This tutorial explains the concept of buffer capacity and how to calculate it, with examples and diagrams. Learn about acidic and alkaline buffers, their preparation, mechanism, and applications with examples and problems. Alkaline buffer solutions are commonly made from a weak base and one of its salts. It is able to neutralize small amounts. How Does An Alkaline Buffer Work.
From www.brainkart.com
How do buffers work? How Does An Alkaline Buffer Work Alkaline buffer solutions are commonly made from a weak base and one of its salts. Learn how buffers protect against ph changes when strong acid or base is added. Alkaline buffer solutions are commonly made. It is able to neutralize small amounts of added acid or base, thus. An alkaline buffer solution has a ph greater than 7. An alkaline. How Does An Alkaline Buffer Work.
From www.numerade.com
SOLVEDWhat is a buffer? How does a buffer work? How does it neutralize How Does An Alkaline Buffer Work Alkaline buffer solutions are commonly made. Learn about acidic and alkaline buffers, their preparation, mechanism, and applications with examples and problems. An alkaline buffer solution has a ph greater than 7. This tutorial explains the concept of buffer capacity and how to calculate it, with examples and diagrams. An alkaline buffer solution has a ph greater than 7. Alkaline buffer. How Does An Alkaline Buffer Work.
From cjarquin.medium.com
Aerogel Buffer Time. Week 10 Reducing Property… by Carlos Manuel How Does An Alkaline Buffer Work An alkaline buffer solution has a ph greater than 7. Learn about acidic and alkaline buffers, their preparation, mechanism, and applications with examples and problems. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Alkaline buffer solutions are commonly made. Alkaline buffer solutions are commonly made from a weak base. How Does An Alkaline Buffer Work.
From giobalfwo.blob.core.windows.net
How Long Does Ph Buffer Last at Betty Gerald blog How Does An Alkaline Buffer Work Alkaline buffer solutions are commonly made from a weak base and one of its salts. Alkaline buffer solutions are commonly made. Learn about acidic and alkaline buffers, their preparation, mechanism, and applications with examples and problems. An alkaline buffer solution has a ph greater than 7. This tutorial explains the concept of buffer capacity and how to calculate it, with. How Does An Alkaline Buffer Work.
From www.youtube.com
More About Buffers (A2 Chemistry) YouTube How Does An Alkaline Buffer Work Alkaline buffer solutions are commonly made from a weak base and one of its salts. Alkaline buffer solutions are commonly made. An alkaline buffer solution has a ph greater than 7. An alkaline buffer solution has a ph greater than 7. It is able to neutralize small amounts of added acid or base, thus. Learn how buffers protect against ph. How Does An Alkaline Buffer Work.
From www.youtube.com
14.10 Buffers Solutions that Resist pH Change YouTube How Does An Alkaline Buffer Work A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Learn about acidic and alkaline buffers, their preparation, mechanism, and applications with examples and problems. Learn how buffers protect against ph changes when strong acid or base is added. Alkaline buffer solutions are commonly made from a weak base and one. How Does An Alkaline Buffer Work.
From www.chemicals.co.uk
What Is A Buffer Solution? The Chemistry Blog How Does An Alkaline Buffer Work This tutorial explains the concept of buffer capacity and how to calculate it, with examples and diagrams. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It is able to neutralize small amounts of added acid or base, thus. Learn about acidic and alkaline buffers, their preparation, mechanism, and applications. How Does An Alkaline Buffer Work.
From animalia-life.club
Phosphate Buffer System Equation How Does An Alkaline Buffer Work Alkaline buffer solutions are commonly made. Alkaline buffer solutions are commonly made from a weak base and one of its salts. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. An alkaline buffer solution has a ph greater than 7. This tutorial explains the concept of buffer capacity and how. How Does An Alkaline Buffer Work.
From store.dafi.us
How Does An Alkaline Water Filter Work? Dafi LLC How Does An Alkaline Buffer Work An alkaline buffer solution has a ph greater than 7. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Alkaline buffer solutions are commonly made from a weak base and one of its salts. Alkaline buffer solutions are commonly made. Learn how buffers protect against ph changes when strong acid. How Does An Alkaline Buffer Work.
From www.youtube.com
How Does a Battery Work? Alkaline Batteries AP Chemistry YouTube How Does An Alkaline Buffer Work It is able to neutralize small amounts of added acid or base, thus. An alkaline buffer solution has a ph greater than 7. Alkaline buffer solutions are commonly made. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Learn about acidic and alkaline buffers, their preparation, mechanism, and applications with. How Does An Alkaline Buffer Work.
From www.slideshare.net
Protein Purification Slides How Does An Alkaline Buffer Work This tutorial explains the concept of buffer capacity and how to calculate it, with examples and diagrams. It is able to neutralize small amounts of added acid or base, thus. Learn how buffers protect against ph changes when strong acid or base is added. Alkaline buffer solutions are commonly made. An alkaline buffer solution has a ph greater than 7.. How Does An Alkaline Buffer Work.
From saylordotorg.github.io
Buffers How Does An Alkaline Buffer Work An alkaline buffer solution has a ph greater than 7. Alkaline buffer solutions are commonly made from a weak base and one of its salts. Learn about acidic and alkaline buffers, their preparation, mechanism, and applications with examples and problems. Alkaline buffer solutions are commonly made. Learn how buffers protect against ph changes when strong acid or base is added.. How Does An Alkaline Buffer Work.
From kyvokedymolyb.modellervefiyatlar.com
Acid Base Buffers How Does An Alkaline Buffer Work Learn how buffers protect against ph changes when strong acid or base is added. Alkaline buffer solutions are commonly made from a weak base and one of its salts. Alkaline buffer solutions are commonly made. An alkaline buffer solution has a ph greater than 7. A buffer is a solution that can resist ph change upon the addition of an. How Does An Alkaline Buffer Work.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID6787517 How Does An Alkaline Buffer Work It is able to neutralize small amounts of added acid or base, thus. An alkaline buffer solution has a ph greater than 7. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. An alkaline buffer solution has a ph greater than 7. Learn how buffers protect against ph changes when. How Does An Alkaline Buffer Work.
From www.youtube.com
Alkaline Phosphatase Enzyme An Overview YouTube How Does An Alkaline Buffer Work A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. This tutorial explains the concept of buffer capacity and how to calculate it, with examples and diagrams. An alkaline buffer solution has a ph greater than 7. An alkaline buffer solution has a ph greater than 7. It is able to. How Does An Alkaline Buffer Work.
From mywaterfilter.com.au
How Does an Alkaline Water Filter Work? How Does An Alkaline Buffer Work This tutorial explains the concept of buffer capacity and how to calculate it, with examples and diagrams. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Learn how buffers protect against ph changes when strong acid or base is added. Alkaline buffer solutions are commonly made. It is able to. How Does An Alkaline Buffer Work.
From www.slideserve.com
PPT Ch. 16 Ionic Equilibria PowerPoint Presentation, free download How Does An Alkaline Buffer Work It is able to neutralize small amounts of added acid or base, thus. Learn about acidic and alkaline buffers, their preparation, mechanism, and applications with examples and problems. An alkaline buffer solution has a ph greater than 7. Learn how buffers protect against ph changes when strong acid or base is added. Alkaline buffer solutions are commonly made. An alkaline. How Does An Alkaline Buffer Work.
From courses.lumenlearning.com
Buffers Chemistry for Majors How Does An Alkaline Buffer Work A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Learn how buffers protect against ph changes when strong acid or base is added. An alkaline buffer solution has a ph greater than 7. This tutorial explains the concept of buffer capacity and how to calculate it, with examples and diagrams.. How Does An Alkaline Buffer Work.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID691391 How Does An Alkaline Buffer Work An alkaline buffer solution has a ph greater than 7. Alkaline buffer solutions are commonly made. Alkaline buffer solutions are commonly made from a weak base and one of its salts. Learn how buffers protect against ph changes when strong acid or base is added. An alkaline buffer solution has a ph greater than 7. A buffer is a solution. How Does An Alkaline Buffer Work.
From waterfilterguru.com
What is an Alkaline Water Filter and How Does it Work? How Does An Alkaline Buffer Work Learn about acidic and alkaline buffers, their preparation, mechanism, and applications with examples and problems. Alkaline buffer solutions are commonly made. This tutorial explains the concept of buffer capacity and how to calculate it, with examples and diagrams. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. An alkaline buffer. How Does An Alkaline Buffer Work.
From www.youtube.com
Acidic and Basic buffer Identification and Preparation Tips and Tricks How Does An Alkaline Buffer Work An alkaline buffer solution has a ph greater than 7. This tutorial explains the concept of buffer capacity and how to calculate it, with examples and diagrams. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Alkaline buffer solutions are commonly made. Learn about acidic and alkaline buffers, their preparation,. How Does An Alkaline Buffer Work.
From design.udlvirtual.edu.pe
How Does A Circular Buffer Work Design Talk How Does An Alkaline Buffer Work It is able to neutralize small amounts of added acid or base, thus. Learn how buffers protect against ph changes when strong acid or base is added. Learn about acidic and alkaline buffers, their preparation, mechanism, and applications with examples and problems. Alkaline buffer solutions are commonly made. Alkaline buffer solutions are commonly made from a weak base and one. How Does An Alkaline Buffer Work.
From www.indieaqua.pt
SEACHEM ALKALINE BUFFER 1,2KG Indie Aqua How Does An Alkaline Buffer Work An alkaline buffer solution has a ph greater than 7. Alkaline buffer solutions are commonly made. This tutorial explains the concept of buffer capacity and how to calculate it, with examples and diagrams. Learn about acidic and alkaline buffers, their preparation, mechanism, and applications with examples and problems. Alkaline buffer solutions are commonly made from a weak base and one. How Does An Alkaline Buffer Work.
From www.coursehero.com
[Solved] 1. Explain what is in a buffer. Discuss the function of a How Does An Alkaline Buffer Work A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. An alkaline buffer solution has a ph greater than 7. Learn how buffers protect against ph changes when strong acid or base is added. An alkaline buffer solution has a ph greater than 7. Alkaline buffer solutions are commonly made from. How Does An Alkaline Buffer Work.
From chansonalkalinewater.com
How an Alkaline Water Ionizer Works Diagram Chanson Water USA How Does An Alkaline Buffer Work An alkaline buffer solution has a ph greater than 7. Alkaline buffer solutions are commonly made from a weak base and one of its salts. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. This tutorial explains the concept of buffer capacity and how to calculate it, with examples and. How Does An Alkaline Buffer Work.
From www.youtube.com
Buffer action in the blood YouTube How Does An Alkaline Buffer Work This tutorial explains the concept of buffer capacity and how to calculate it, with examples and diagrams. An alkaline buffer solution has a ph greater than 7. Alkaline buffer solutions are commonly made. Learn about acidic and alkaline buffers, their preparation, mechanism, and applications with examples and problems. A buffer is a solution that can resist ph change upon the. How Does An Alkaline Buffer Work.
From scienceinfo.com
Buffer Solution Types, Properties, and Uses How Does An Alkaline Buffer Work Learn about acidic and alkaline buffers, their preparation, mechanism, and applications with examples and problems. Learn how buffers protect against ph changes when strong acid or base is added. This tutorial explains the concept of buffer capacity and how to calculate it, with examples and diagrams. Alkaline buffer solutions are commonly made. It is able to neutralize small amounts of. How Does An Alkaline Buffer Work.
From www.osmosis.org
Making buffer solutions Osmosis How Does An Alkaline Buffer Work Learn how buffers protect against ph changes when strong acid or base is added. An alkaline buffer solution has a ph greater than 7. It is able to neutralize small amounts of added acid or base, thus. Alkaline buffer solutions are commonly made from a weak base and one of its salts. This tutorial explains the concept of buffer capacity. How Does An Alkaline Buffer Work.
From teslahealthylife.com
How Does An Alkaline Water Filter Work? How Does An Alkaline Buffer Work A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. An alkaline buffer solution has a ph greater than 7. Learn how buffers protect against ph changes when strong acid or base is added. Learn about acidic and alkaline buffers, their preparation, mechanism, and applications with examples and problems. This tutorial. How Does An Alkaline Buffer Work.
From chemistryguru.com.sg
What is a Buffer Solution? How Does An Alkaline Buffer Work Alkaline buffer solutions are commonly made. It is able to neutralize small amounts of added acid or base, thus. This tutorial explains the concept of buffer capacity and how to calculate it, with examples and diagrams. Learn how buffers protect against ph changes when strong acid or base is added. Alkaline buffer solutions are commonly made from a weak base. How Does An Alkaline Buffer Work.
From www.slideserve.com
PPT Water & Buffers PowerPoint Presentation, free download ID3886735 How Does An Alkaline Buffer Work It is able to neutralize small amounts of added acid or base, thus. Alkaline buffer solutions are commonly made from a weak base and one of its salts. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. An alkaline buffer solution has a ph greater than 7. This tutorial explains. How Does An Alkaline Buffer Work.
From www.geeksforgeeks.org
Buffer Solution Definition, Types, Formula, Examples, and FAQs How Does An Alkaline Buffer Work Learn how buffers protect against ph changes when strong acid or base is added. Alkaline buffer solutions are commonly made. It is able to neutralize small amounts of added acid or base, thus. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. An alkaline buffer solution has a ph greater. How Does An Alkaline Buffer Work.