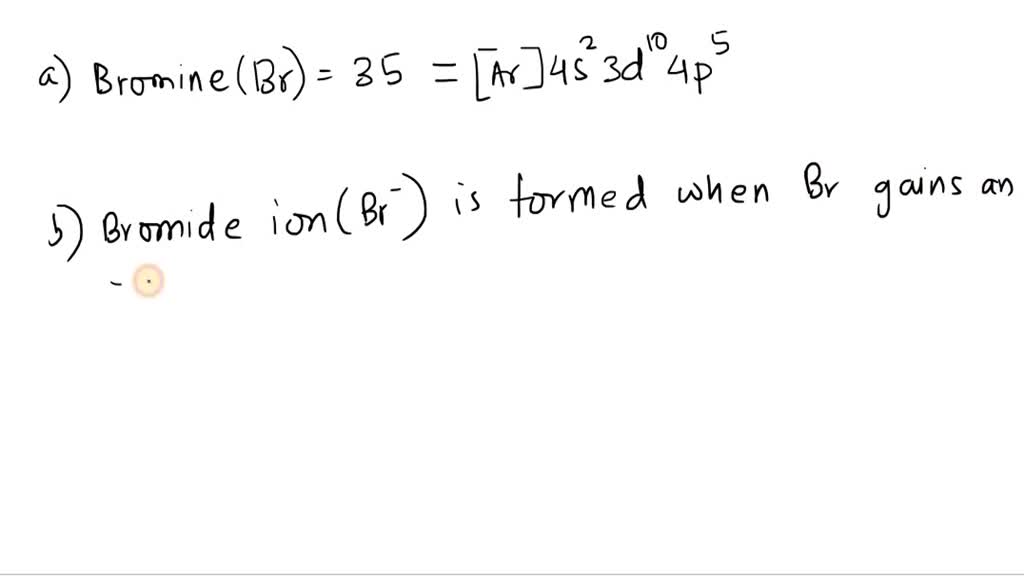A Bromine Atom In An Excited State . An excited state is an energy level of an atom, ion, or molecule in which an electron is at a higher energy level than its ground state. In summary, we presented the first direct dynamical evidence of roaming for photodissociation of a triatomic molecule originating from a highly excited. An excited state means that (typically) the valence electron has moved from its ground state orbital (i.e. Wavelength selective photochemical reactions of excited 2 p 1/2 bromine atoms are found to occur. In an excited state, an electron from. To consider what happens in the process of fluorescence, we need to think of the possible energy states for a ground and excited state.
from www.animalia-life.club
To consider what happens in the process of fluorescence, we need to think of the possible energy states for a ground and excited state. Wavelength selective photochemical reactions of excited 2 p 1/2 bromine atoms are found to occur. In summary, we presented the first direct dynamical evidence of roaming for photodissociation of a triatomic molecule originating from a highly excited. In an excited state, an electron from. An excited state means that (typically) the valence electron has moved from its ground state orbital (i.e. An excited state is an energy level of an atom, ion, or molecule in which an electron is at a higher energy level than its ground state.
Electron Configuration For Bromine
A Bromine Atom In An Excited State To consider what happens in the process of fluorescence, we need to think of the possible energy states for a ground and excited state. To consider what happens in the process of fluorescence, we need to think of the possible energy states for a ground and excited state. An excited state means that (typically) the valence electron has moved from its ground state orbital (i.e. In summary, we presented the first direct dynamical evidence of roaming for photodissociation of a triatomic molecule originating from a highly excited. In an excited state, an electron from. Wavelength selective photochemical reactions of excited 2 p 1/2 bromine atoms are found to occur. An excited state is an energy level of an atom, ion, or molecule in which an electron is at a higher energy level than its ground state.
From www.sciencephoto.com
Bromine, atomic structure Stock Image C018/3716 Science Photo Library A Bromine Atom In An Excited State An excited state is an energy level of an atom, ion, or molecule in which an electron is at a higher energy level than its ground state. An excited state means that (typically) the valence electron has moved from its ground state orbital (i.e. Wavelength selective photochemical reactions of excited 2 p 1/2 bromine atoms are found to occur. In. A Bromine Atom In An Excited State.
From www.dreamstime.com
Bromine stock illustration. Illustration of render, formula 139651101 A Bromine Atom In An Excited State An excited state means that (typically) the valence electron has moved from its ground state orbital (i.e. In an excited state, an electron from. Wavelength selective photochemical reactions of excited 2 p 1/2 bromine atoms are found to occur. In summary, we presented the first direct dynamical evidence of roaming for photodissociation of a triatomic molecule originating from a highly. A Bromine Atom In An Excited State.
From ar.inspiredpencil.com
Atomic Structure Of Bromine A Bromine Atom In An Excited State In an excited state, an electron from. An excited state is an energy level of an atom, ion, or molecule in which an electron is at a higher energy level than its ground state. Wavelength selective photochemical reactions of excited 2 p 1/2 bromine atoms are found to occur. To consider what happens in the process of fluorescence, we need. A Bromine Atom In An Excited State.
From www.alamy.com
Bromine atom, with mass and energy levels. Vector illustration Stock A Bromine Atom In An Excited State Wavelength selective photochemical reactions of excited 2 p 1/2 bromine atoms are found to occur. In an excited state, an electron from. To consider what happens in the process of fluorescence, we need to think of the possible energy states for a ground and excited state. An excited state is an energy level of an atom, ion, or molecule in. A Bromine Atom In An Excited State.
From www.alamy.com
Bromine Atom Shell Stock Vector Image & Art Alamy A Bromine Atom In An Excited State In an excited state, an electron from. Wavelength selective photochemical reactions of excited 2 p 1/2 bromine atoms are found to occur. An excited state is an energy level of an atom, ion, or molecule in which an electron is at a higher energy level than its ground state. An excited state means that (typically) the valence electron has moved. A Bromine Atom In An Excited State.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram A Bromine Atom In An Excited State Wavelength selective photochemical reactions of excited 2 p 1/2 bromine atoms are found to occur. To consider what happens in the process of fluorescence, we need to think of the possible energy states for a ground and excited state. In summary, we presented the first direct dynamical evidence of roaming for photodissociation of a triatomic molecule originating from a highly. A Bromine Atom In An Excited State.
From www.animalia-life.club
Electron Configuration For Bromine A Bromine Atom In An Excited State An excited state means that (typically) the valence electron has moved from its ground state orbital (i.e. An excited state is an energy level of an atom, ion, or molecule in which an electron is at a higher energy level than its ground state. In summary, we presented the first direct dynamical evidence of roaming for photodissociation of a triatomic. A Bromine Atom In An Excited State.
From www.alamy.com
Bromine (Br). Diagram of the nuclear composition, electron A Bromine Atom In An Excited State An excited state is an energy level of an atom, ion, or molecule in which an electron is at a higher energy level than its ground state. In an excited state, an electron from. In summary, we presented the first direct dynamical evidence of roaming for photodissociation of a triatomic molecule originating from a highly excited. To consider what happens. A Bromine Atom In An Excited State.
From sciencenotes.org
Bromine Facts Atomic Number 35 and Element Symbol Br A Bromine Atom In An Excited State Wavelength selective photochemical reactions of excited 2 p 1/2 bromine atoms are found to occur. In summary, we presented the first direct dynamical evidence of roaming for photodissociation of a triatomic molecule originating from a highly excited. To consider what happens in the process of fluorescence, we need to think of the possible energy states for a ground and excited. A Bromine Atom In An Excited State.
From material-properties.org
Bromine Periodic Table and Atomic Properties A Bromine Atom In An Excited State In summary, we presented the first direct dynamical evidence of roaming for photodissociation of a triatomic molecule originating from a highly excited. An excited state means that (typically) the valence electron has moved from its ground state orbital (i.e. To consider what happens in the process of fluorescence, we need to think of the possible energy states for a ground. A Bromine Atom In An Excited State.
From ar.inspiredpencil.com
Atomic Structure Of Bromine A Bromine Atom In An Excited State To consider what happens in the process of fluorescence, we need to think of the possible energy states for a ground and excited state. An excited state means that (typically) the valence electron has moved from its ground state orbital (i.e. In an excited state, an electron from. In summary, we presented the first direct dynamical evidence of roaming for. A Bromine Atom In An Excited State.
From ar.inspiredpencil.com
Atomic Structure Of Bromine A Bromine Atom In An Excited State In summary, we presented the first direct dynamical evidence of roaming for photodissociation of a triatomic molecule originating from a highly excited. An excited state is an energy level of an atom, ion, or molecule in which an electron is at a higher energy level than its ground state. An excited state means that (typically) the valence electron has moved. A Bromine Atom In An Excited State.
From www.vectorstock.com
Br2 bromine molecule Royalty Free Vector Image A Bromine Atom In An Excited State In summary, we presented the first direct dynamical evidence of roaming for photodissociation of a triatomic molecule originating from a highly excited. To consider what happens in the process of fluorescence, we need to think of the possible energy states for a ground and excited state. An excited state means that (typically) the valence electron has moved from its ground. A Bromine Atom In An Excited State.
From jazlyndesnhwu.blogspot.com
Excited State Electron Configuration of Bromine A Bromine Atom In An Excited State Wavelength selective photochemical reactions of excited 2 p 1/2 bromine atoms are found to occur. An excited state means that (typically) the valence electron has moved from its ground state orbital (i.e. In an excited state, an electron from. In summary, we presented the first direct dynamical evidence of roaming for photodissociation of a triatomic molecule originating from a highly. A Bromine Atom In An Excited State.
From www.animalia-life.club
Electron Configuration For Bromine A Bromine Atom In An Excited State An excited state is an energy level of an atom, ion, or molecule in which an electron is at a higher energy level than its ground state. In summary, we presented the first direct dynamical evidence of roaming for photodissociation of a triatomic molecule originating from a highly excited. Wavelength selective photochemical reactions of excited 2 p 1/2 bromine atoms. A Bromine Atom In An Excited State.
From www.animalia-life.club
Electron Configuration For Bromine A Bromine Atom In An Excited State In summary, we presented the first direct dynamical evidence of roaming for photodissociation of a triatomic molecule originating from a highly excited. An excited state means that (typically) the valence electron has moved from its ground state orbital (i.e. An excited state is an energy level of an atom, ion, or molecule in which an electron is at a higher. A Bromine Atom In An Excited State.
From cepvgsav.blob.core.windows.net
Bromine Molecule Lewis Structure at Therese Boyd blog A Bromine Atom In An Excited State In summary, we presented the first direct dynamical evidence of roaming for photodissociation of a triatomic molecule originating from a highly excited. An excited state means that (typically) the valence electron has moved from its ground state orbital (i.e. In an excited state, an electron from. An excited state is an energy level of an atom, ion, or molecule in. A Bromine Atom In An Excited State.
From www.animalia-life.club
Electron Configuration For Bromine A Bromine Atom In An Excited State In summary, we presented the first direct dynamical evidence of roaming for photodissociation of a triatomic molecule originating from a highly excited. An excited state means that (typically) the valence electron has moved from its ground state orbital (i.e. Wavelength selective photochemical reactions of excited 2 p 1/2 bromine atoms are found to occur. In an excited state, an electron. A Bromine Atom In An Excited State.
From valenceelectrons.com
Complete Electron Configuration for Bromine (Br, Br ion) A Bromine Atom In An Excited State In an excited state, an electron from. In summary, we presented the first direct dynamical evidence of roaming for photodissociation of a triatomic molecule originating from a highly excited. An excited state means that (typically) the valence electron has moved from its ground state orbital (i.e. To consider what happens in the process of fluorescence, we need to think of. A Bromine Atom In An Excited State.
From www.numerade.com
SOLVED A bromine atom in the excited state could have an electron A Bromine Atom In An Excited State Wavelength selective photochemical reactions of excited 2 p 1/2 bromine atoms are found to occur. An excited state is an energy level of an atom, ion, or molecule in which an electron is at a higher energy level than its ground state. To consider what happens in the process of fluorescence, we need to think of the possible energy states. A Bromine Atom In An Excited State.
From www.dreamstime.com
Element of Bromine stock vector. Illustration of college 104400483 A Bromine Atom In An Excited State To consider what happens in the process of fluorescence, we need to think of the possible energy states for a ground and excited state. In summary, we presented the first direct dynamical evidence of roaming for photodissociation of a triatomic molecule originating from a highly excited. In an excited state, an electron from. Wavelength selective photochemical reactions of excited 2. A Bromine Atom In An Excited State.
From www.nuclear-power.com
Bromine Electron Affinity Electronegativity Ionization Energy of A Bromine Atom In An Excited State An excited state means that (typically) the valence electron has moved from its ground state orbital (i.e. In summary, we presented the first direct dynamical evidence of roaming for photodissociation of a triatomic molecule originating from a highly excited. To consider what happens in the process of fluorescence, we need to think of the possible energy states for a ground. A Bromine Atom In An Excited State.
From www.alamy.com
Bromine molecule Br2 Stock Photo Alamy A Bromine Atom In An Excited State Wavelength selective photochemical reactions of excited 2 p 1/2 bromine atoms are found to occur. In an excited state, an electron from. To consider what happens in the process of fluorescence, we need to think of the possible energy states for a ground and excited state. An excited state is an energy level of an atom, ion, or molecule in. A Bromine Atom In An Excited State.
From mungfali.com
Aufbau Diagram For Bromine A Bromine Atom In An Excited State An excited state means that (typically) the valence electron has moved from its ground state orbital (i.e. Wavelength selective photochemical reactions of excited 2 p 1/2 bromine atoms are found to occur. In an excited state, an electron from. In summary, we presented the first direct dynamical evidence of roaming for photodissociation of a triatomic molecule originating from a highly. A Bromine Atom In An Excited State.
From www.animalia-life.club
Electron Configuration For Bromine A Bromine Atom In An Excited State An excited state is an energy level of an atom, ion, or molecule in which an electron is at a higher energy level than its ground state. To consider what happens in the process of fluorescence, we need to think of the possible energy states for a ground and excited state. An excited state means that (typically) the valence electron. A Bromine Atom In An Excited State.
From www.thoughtco.com
Atoms Diagrams Electron Configurations of Elements A Bromine Atom In An Excited State In an excited state, an electron from. To consider what happens in the process of fluorescence, we need to think of the possible energy states for a ground and excited state. Wavelength selective photochemical reactions of excited 2 p 1/2 bromine atoms are found to occur. In summary, we presented the first direct dynamical evidence of roaming for photodissociation of. A Bromine Atom In An Excited State.
From www.pinterest.com
Excited State Easy Science Easy science, Electron configuration, Ap A Bromine Atom In An Excited State In an excited state, an electron from. An excited state means that (typically) the valence electron has moved from its ground state orbital (i.e. Wavelength selective photochemical reactions of excited 2 p 1/2 bromine atoms are found to occur. In summary, we presented the first direct dynamical evidence of roaming for photodissociation of a triatomic molecule originating from a highly. A Bromine Atom In An Excited State.
From ar.inspiredpencil.com
Atomic Structure Of Bromine A Bromine Atom In An Excited State To consider what happens in the process of fluorescence, we need to think of the possible energy states for a ground and excited state. In an excited state, an electron from. Wavelength selective photochemical reactions of excited 2 p 1/2 bromine atoms are found to occur. In summary, we presented the first direct dynamical evidence of roaming for photodissociation of. A Bromine Atom In An Excited State.
From ar.inspiredpencil.com
Atomic Structure Of Bromine A Bromine Atom In An Excited State Wavelength selective photochemical reactions of excited 2 p 1/2 bromine atoms are found to occur. To consider what happens in the process of fluorescence, we need to think of the possible energy states for a ground and excited state. An excited state is an energy level of an atom, ion, or molecule in which an electron is at a higher. A Bromine Atom In An Excited State.
From www.alamy.com
Symbol and electron diagram for Bromine Stock Vector Image & Art Alamy A Bromine Atom In An Excited State In an excited state, an electron from. In summary, we presented the first direct dynamical evidence of roaming for photodissociation of a triatomic molecule originating from a highly excited. An excited state means that (typically) the valence electron has moved from its ground state orbital (i.e. Wavelength selective photochemical reactions of excited 2 p 1/2 bromine atoms are found to. A Bromine Atom In An Excited State.
From www.webelements.com
Elements Periodic Table » Bromine » properties of free atoms A Bromine Atom In An Excited State An excited state is an energy level of an atom, ion, or molecule in which an electron is at a higher energy level than its ground state. In an excited state, an electron from. To consider what happens in the process of fluorescence, we need to think of the possible energy states for a ground and excited state. Wavelength selective. A Bromine Atom In An Excited State.
From www.examples.com
Bromine (Br) Definition, Preparation, Properties, Uses, Compounds A Bromine Atom In An Excited State In summary, we presented the first direct dynamical evidence of roaming for photodissociation of a triatomic molecule originating from a highly excited. Wavelength selective photochemical reactions of excited 2 p 1/2 bromine atoms are found to occur. To consider what happens in the process of fluorescence, we need to think of the possible energy states for a ground and excited. A Bromine Atom In An Excited State.
From www.alamy.com
Symbol and electron diagram for Bromine illustration Stock Vector Image A Bromine Atom In An Excited State An excited state is an energy level of an atom, ion, or molecule in which an electron is at a higher energy level than its ground state. Wavelength selective photochemical reactions of excited 2 p 1/2 bromine atoms are found to occur. In summary, we presented the first direct dynamical evidence of roaming for photodissociation of a triatomic molecule originating. A Bromine Atom In An Excited State.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram A Bromine Atom In An Excited State An excited state means that (typically) the valence electron has moved from its ground state orbital (i.e. In an excited state, an electron from. Wavelength selective photochemical reactions of excited 2 p 1/2 bromine atoms are found to occur. In summary, we presented the first direct dynamical evidence of roaming for photodissociation of a triatomic molecule originating from a highly. A Bromine Atom In An Excited State.
From www.slideserve.com
PPT Electron Configuration Ions and Excite State PowerPoint A Bromine Atom In An Excited State An excited state is an energy level of an atom, ion, or molecule in which an electron is at a higher energy level than its ground state. An excited state means that (typically) the valence electron has moved from its ground state orbital (i.e. In an excited state, an electron from. In summary, we presented the first direct dynamical evidence. A Bromine Atom In An Excited State.