Titration Experiment Test. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a. © royal society of chemistry 2024 registered charity number: This resource has been developed in partnership with learning science and the university of bristol. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or base. For the best experience we. 207890 this website collects cookies to deliver a better user experience. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution. Be able to determine the molar mass of a. Includes kit list and safety instructions.
from mungfali.com
Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. For the best experience we. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or base. Includes kit list and safety instructions. © royal society of chemistry 2024 registered charity number: Be able to determine the molar mass of a. This resource has been developed in partnership with learning science and the university of bristol. A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution.
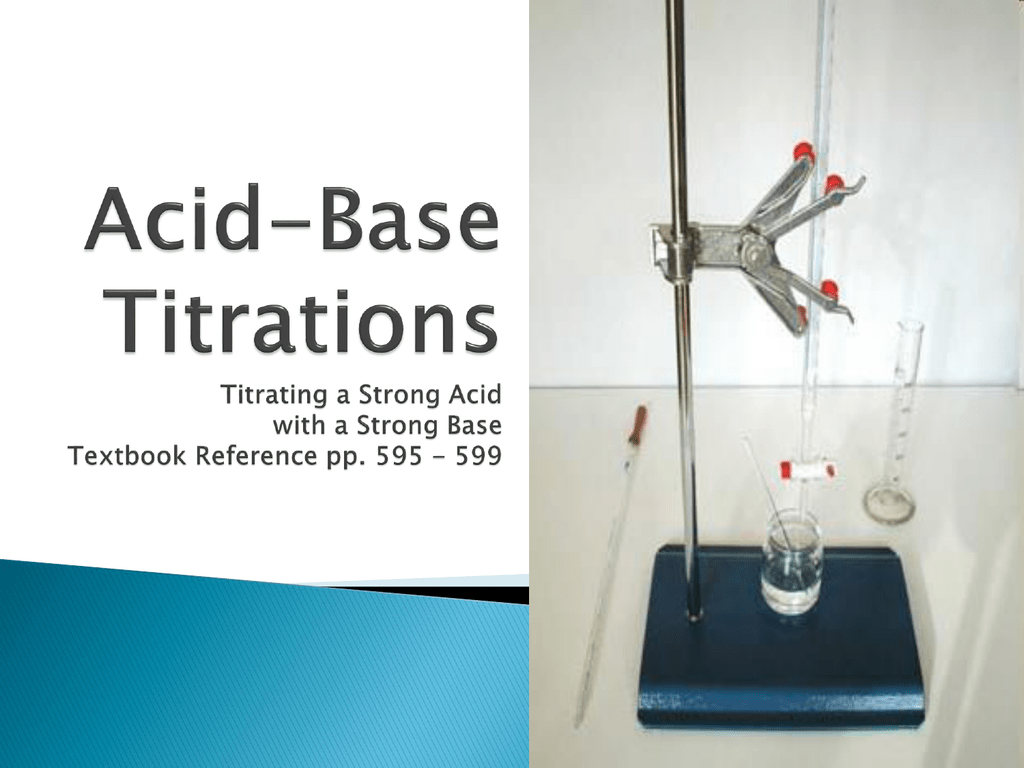
Acid Base Titration Lab
Titration Experiment Test For the best experience we. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. 207890 this website collects cookies to deliver a better user experience. For the best experience we. © royal society of chemistry 2024 registered charity number: A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution. This resource has been developed in partnership with learning science and the university of bristol. Includes kit list and safety instructions. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or base. Be able to determine the molar mass of a. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown.
