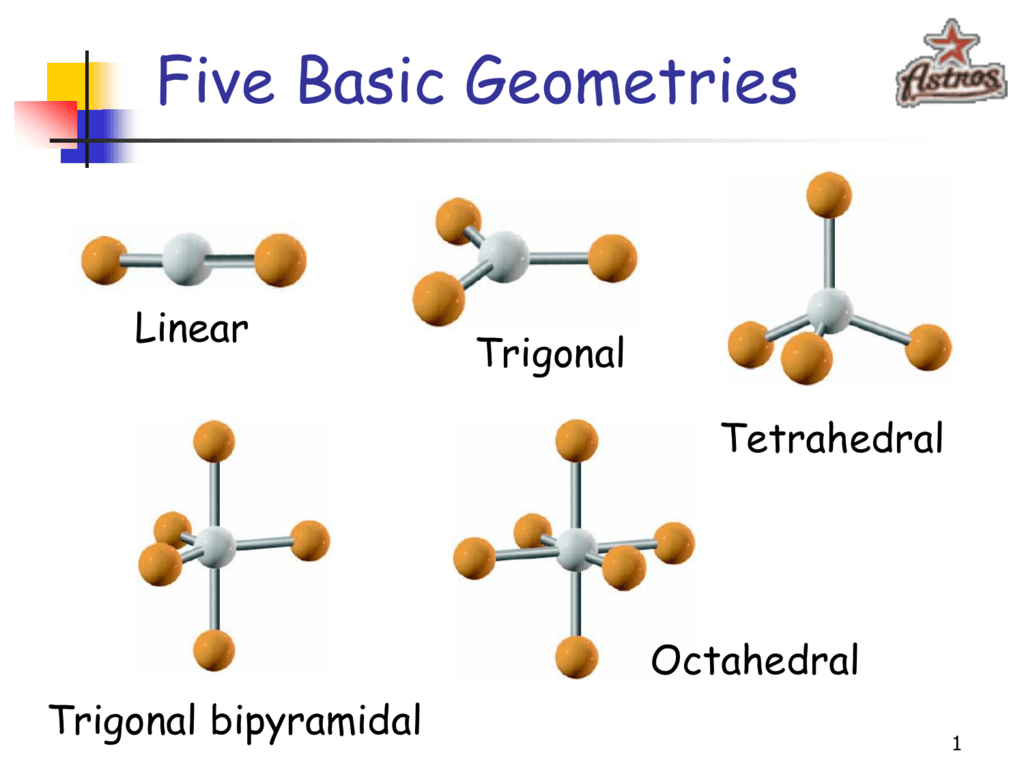
Linear Example Molecules . The atoms are arranged in a straight line, and the angle between the bonds is 180 °. It specifies the geometry shaped by a central atom surrounded by two other atoms. Examples of molecules with linear geometry are carbon dioxide (co 2), beryllium chloride (becl 2), and nitric oxide (no). It refers to the geometry shaped by a central atom surrounded by two other atoms. Understanding the molecular structure of a. A molecule with four electron groups about the central atom but only one electron group bonded to another atom is linear because there are only. If a molecule contains only two atoms, those two atoms are in a straight line and thus form a linear molecule. The atoms are arranged in a straight line, and the angle between the bonds, or bond.
from studylib.net
It specifies the geometry shaped by a central atom surrounded by two other atoms. The atoms are arranged in a straight line, and the angle between the bonds is 180 °. Examples of molecules with linear geometry are carbon dioxide (co 2), beryllium chloride (becl 2), and nitric oxide (no). If a molecule contains only two atoms, those two atoms are in a straight line and thus form a linear molecule. It refers to the geometry shaped by a central atom surrounded by two other atoms. A molecule with four electron groups about the central atom but only one electron group bonded to another atom is linear because there are only. The atoms are arranged in a straight line, and the angle between the bonds, or bond. Understanding the molecular structure of a.
Chapter One
Linear Example Molecules Examples of molecules with linear geometry are carbon dioxide (co 2), beryllium chloride (becl 2), and nitric oxide (no). The atoms are arranged in a straight line, and the angle between the bonds is 180 °. It specifies the geometry shaped by a central atom surrounded by two other atoms. Examples of molecules with linear geometry are carbon dioxide (co 2), beryllium chloride (becl 2), and nitric oxide (no). If a molecule contains only two atoms, those two atoms are in a straight line and thus form a linear molecule. Understanding the molecular structure of a. It refers to the geometry shaped by a central atom surrounded by two other atoms. A molecule with four electron groups about the central atom but only one electron group bonded to another atom is linear because there are only. The atoms are arranged in a straight line, and the angle between the bonds, or bond.
From www.webassign.net
Chapter 6 Molecular Structure Linear Example Molecules Understanding the molecular structure of a. It specifies the geometry shaped by a central atom surrounded by two other atoms. The atoms are arranged in a straight line, and the angle between the bonds is 180 °. If a molecule contains only two atoms, those two atoms are in a straight line and thus form a linear molecule. Examples of. Linear Example Molecules.
From chem.libretexts.org
9.7 The Shapes of Molecules Chemistry LibreTexts Linear Example Molecules If a molecule contains only two atoms, those two atoms are in a straight line and thus form a linear molecule. A molecule with four electron groups about the central atom but only one electron group bonded to another atom is linear because there are only. Examples of molecules with linear geometry are carbon dioxide (co 2), beryllium chloride (becl. Linear Example Molecules.
From www.slideserve.com
PPT Drawing Lewis structures PowerPoint Presentation, free download Linear Example Molecules It refers to the geometry shaped by a central atom surrounded by two other atoms. The atoms are arranged in a straight line, and the angle between the bonds, or bond. Understanding the molecular structure of a. The atoms are arranged in a straight line, and the angle between the bonds is 180 °. A molecule with four electron groups. Linear Example Molecules.
From chemistryguru.com.sg
VSEPR Theory and Shapes of Molecules Linear Example Molecules Understanding the molecular structure of a. Examples of molecules with linear geometry are carbon dioxide (co 2), beryllium chloride (becl 2), and nitric oxide (no). It refers to the geometry shaped by a central atom surrounded by two other atoms. If a molecule contains only two atoms, those two atoms are in a straight line and thus form a linear. Linear Example Molecules.
From compositeskn.org
Polymer (matrix) structure A236 CKN Knowledge in Practice Centre Linear Example Molecules The atoms are arranged in a straight line, and the angle between the bonds is 180 °. It refers to the geometry shaped by a central atom surrounded by two other atoms. Examples of molecules with linear geometry are carbon dioxide (co 2), beryllium chloride (becl 2), and nitric oxide (no). If a molecule contains only two atoms, those two. Linear Example Molecules.
From www.siyavula.com
3.2 Molecular shape Atomic combinations Siyavula Linear Example Molecules Understanding the molecular structure of a. The atoms are arranged in a straight line, and the angle between the bonds is 180 °. It specifies the geometry shaped by a central atom surrounded by two other atoms. Examples of molecules with linear geometry are carbon dioxide (co 2), beryllium chloride (becl 2), and nitric oxide (no). It refers to the. Linear Example Molecules.
From studylib.net
Chapter One Linear Example Molecules The atoms are arranged in a straight line, and the angle between the bonds is 180 °. It specifies the geometry shaped by a central atom surrounded by two other atoms. If a molecule contains only two atoms, those two atoms are in a straight line and thus form a linear molecule. Understanding the molecular structure of a. Examples of. Linear Example Molecules.
From www.dreamstime.com
Molecular Geometry Structure of Elements Stock Vector Illustration of Linear Example Molecules Examples of molecules with linear geometry are carbon dioxide (co 2), beryllium chloride (becl 2), and nitric oxide (no). A molecule with four electron groups about the central atom but only one electron group bonded to another atom is linear because there are only. Understanding the molecular structure of a. It refers to the geometry shaped by a central atom. Linear Example Molecules.
From www.differencebetween.com
Difference Between Linear and Bent Molecules Compare the Difference Linear Example Molecules The atoms are arranged in a straight line, and the angle between the bonds is 180 °. The atoms are arranged in a straight line, and the angle between the bonds, or bond. Understanding the molecular structure of a. It specifies the geometry shaped by a central atom surrounded by two other atoms. It refers to the geometry shaped by. Linear Example Molecules.
From www.priyamstudycentre.com
Molecule Definition, Examples, Structure, Hybridization Linear Example Molecules It specifies the geometry shaped by a central atom surrounded by two other atoms. Examples of molecules with linear geometry are carbon dioxide (co 2), beryllium chloride (becl 2), and nitric oxide (no). A molecule with four electron groups about the central atom but only one electron group bonded to another atom is linear because there are only. If a. Linear Example Molecules.
From www.sciencefacts.net
Molecule Definition, Examples, Facts & Diagram Linear Example Molecules Understanding the molecular structure of a. It specifies the geometry shaped by a central atom surrounded by two other atoms. If a molecule contains only two atoms, those two atoms are in a straight line and thus form a linear molecule. The atoms are arranged in a straight line, and the angle between the bonds, or bond. The atoms are. Linear Example Molecules.
From www.chemistrysteps.com
VSEPR Theory Geometry of Organic Molecules Chemistry Steps Linear Example Molecules Understanding the molecular structure of a. If a molecule contains only two atoms, those two atoms are in a straight line and thus form a linear molecule. The atoms are arranged in a straight line, and the angle between the bonds is 180 °. It refers to the geometry shaped by a central atom surrounded by two other atoms. It. Linear Example Molecules.
From sciencenotes.org
What Is a Molecule? Definition and Examples Linear Example Molecules The atoms are arranged in a straight line, and the angle between the bonds is 180 °. Examples of molecules with linear geometry are carbon dioxide (co 2), beryllium chloride (becl 2), and nitric oxide (no). If a molecule contains only two atoms, those two atoms are in a straight line and thus form a linear molecule. It refers to. Linear Example Molecules.
From www.differencebetween.com
Difference Between Linear and Molecules Compare the Linear Example Molecules It specifies the geometry shaped by a central atom surrounded by two other atoms. If a molecule contains only two atoms, those two atoms are in a straight line and thus form a linear molecule. A molecule with four electron groups about the central atom but only one electron group bonded to another atom is linear because there are only.. Linear Example Molecules.
From www.slideserve.com
PPT CHEM 515 Spectroscopy PowerPoint Presentation, free download ID Linear Example Molecules It specifies the geometry shaped by a central atom surrounded by two other atoms. The atoms are arranged in a straight line, and the angle between the bonds is 180 °. It refers to the geometry shaped by a central atom surrounded by two other atoms. Examples of molecules with linear geometry are carbon dioxide (co 2), beryllium chloride (becl. Linear Example Molecules.
From cartridges.esciencelabs.com
Diagram of shapes of molecules, showng bonding pairs, arrangement of Linear Example Molecules Understanding the molecular structure of a. A molecule with four electron groups about the central atom but only one electron group bonded to another atom is linear because there are only. It refers to the geometry shaped by a central atom surrounded by two other atoms. Examples of molecules with linear geometry are carbon dioxide (co 2), beryllium chloride (becl. Linear Example Molecules.
From daftsex-hd.com
Linear Chemical Formula Examples Chemical Info DaftSex HD Linear Example Molecules It refers to the geometry shaped by a central atom surrounded by two other atoms. The atoms are arranged in a straight line, and the angle between the bonds, or bond. It specifies the geometry shaped by a central atom surrounded by two other atoms. Examples of molecules with linear geometry are carbon dioxide (co 2), beryllium chloride (becl 2),. Linear Example Molecules.
From www.coursehero.com
Molecular Geometry Boundless Chemistry Course Hero Linear Example Molecules Understanding the molecular structure of a. The atoms are arranged in a straight line, and the angle between the bonds is 180 °. It refers to the geometry shaped by a central atom surrounded by two other atoms. The atoms are arranged in a straight line, and the angle between the bonds, or bond. If a molecule contains only two. Linear Example Molecules.
From courses.lumenlearning.com
Reading Covalent Bonds Biology I Linear Example Molecules A molecule with four electron groups about the central atom but only one electron group bonded to another atom is linear because there are only. It specifies the geometry shaped by a central atom surrounded by two other atoms. Understanding the molecular structure of a. The atoms are arranged in a straight line, and the angle between the bonds is. Linear Example Molecules.
From www.masterorganicchemistry.com
Lewis Structures Master Organic Chemistry Linear Example Molecules Examples of molecules with linear geometry are carbon dioxide (co 2), beryllium chloride (becl 2), and nitric oxide (no). The atoms are arranged in a straight line, and the angle between the bonds, or bond. The atoms are arranged in a straight line, and the angle between the bonds is 180 °. It refers to the geometry shaped by a. Linear Example Molecules.
From www.slideserve.com
PPT The Shapes of Molecules PowerPoint Presentation, free download Linear Example Molecules A molecule with four electron groups about the central atom but only one electron group bonded to another atom is linear because there are only. If a molecule contains only two atoms, those two atoms are in a straight line and thus form a linear molecule. It refers to the geometry shaped by a central atom surrounded by two other. Linear Example Molecules.
From www.kompas.com
Notasi VSEPR Pengertian, Rumus, dan Bentuk Molekulnya Linear Example Molecules Understanding the molecular structure of a. It specifies the geometry shaped by a central atom surrounded by two other atoms. If a molecule contains only two atoms, those two atoms are in a straight line and thus form a linear molecule. It refers to the geometry shaped by a central atom surrounded by two other atoms. The atoms are arranged. Linear Example Molecules.
From www.sciencephoto.com
Linear molecule, illustration Stock Image C026/2866 Science Photo Linear Example Molecules It refers to the geometry shaped by a central atom surrounded by two other atoms. The atoms are arranged in a straight line, and the angle between the bonds, or bond. If a molecule contains only two atoms, those two atoms are in a straight line and thus form a linear molecule. It specifies the geometry shaped by a central. Linear Example Molecules.
From www.indiapicturebudget.com
Shape of Molecules infographic diagram including linear trigonal planar Linear Example Molecules Examples of molecules with linear geometry are carbon dioxide (co 2), beryllium chloride (becl 2), and nitric oxide (no). A molecule with four electron groups about the central atom but only one electron group bonded to another atom is linear because there are only. Understanding the molecular structure of a. The atoms are arranged in a straight line, and the. Linear Example Molecules.
From quizlet.com
shapes of molecules Diagram Quizlet Linear Example Molecules Understanding the molecular structure of a. It refers to the geometry shaped by a central atom surrounded by two other atoms. It specifies the geometry shaped by a central atom surrounded by two other atoms. The atoms are arranged in a straight line, and the angle between the bonds, or bond. Examples of molecules with linear geometry are carbon dioxide. Linear Example Molecules.
From www.researchgate.net
Examples of linear and hydrogenbonded complexes studied Linear Example Molecules It specifies the geometry shaped by a central atom surrounded by two other atoms. The atoms are arranged in a straight line, and the angle between the bonds is 180 °. Examples of molecules with linear geometry are carbon dioxide (co 2), beryllium chloride (becl 2), and nitric oxide (no). A molecule with four electron groups about the central atom. Linear Example Molecules.
From www.youtube.com
Linear Molecular Geometry/Shape and Bond Angles YouTube Linear Example Molecules If a molecule contains only two atoms, those two atoms are in a straight line and thus form a linear molecule. A molecule with four electron groups about the central atom but only one electron group bonded to another atom is linear because there are only. It refers to the geometry shaped by a central atom surrounded by two other. Linear Example Molecules.
From www.slideserve.com
PPT Chemistry 545 Chemistry Lecture 1. PowerPoint Linear Example Molecules It specifies the geometry shaped by a central atom surrounded by two other atoms. If a molecule contains only two atoms, those two atoms are in a straight line and thus form a linear molecule. Examples of molecules with linear geometry are carbon dioxide (co 2), beryllium chloride (becl 2), and nitric oxide (no). A molecule with four electron groups. Linear Example Molecules.
From www.slideserve.com
PPT Shapes of Molecules PowerPoint Presentation, free download ID Linear Example Molecules If a molecule contains only two atoms, those two atoms are in a straight line and thus form a linear molecule. A molecule with four electron groups about the central atom but only one electron group bonded to another atom is linear because there are only. Understanding the molecular structure of a. It refers to the geometry shaped by a. Linear Example Molecules.
From www.slideserve.com
PPT Chemical Bonding and VSEPR PowerPoint Presentation, free download Linear Example Molecules The atoms are arranged in a straight line, and the angle between the bonds, or bond. It refers to the geometry shaped by a central atom surrounded by two other atoms. It specifies the geometry shaped by a central atom surrounded by two other atoms. Examples of molecules with linear geometry are carbon dioxide (co 2), beryllium chloride (becl 2),. Linear Example Molecules.
From www.alamy.com
Linear molecule. Computer illustration representing the arrangement of Linear Example Molecules If a molecule contains only two atoms, those two atoms are in a straight line and thus form a linear molecule. Understanding the molecular structure of a. A molecule with four electron groups about the central atom but only one electron group bonded to another atom is linear because there are only. It refers to the geometry shaped by a. Linear Example Molecules.
From chemistryclinic.co.uk
Shapes of simple molecules Chemistry Linear Example Molecules Examples of molecules with linear geometry are carbon dioxide (co 2), beryllium chloride (becl 2), and nitric oxide (no). A molecule with four electron groups about the central atom but only one electron group bonded to another atom is linear because there are only. It refers to the geometry shaped by a central atom surrounded by two other atoms. It. Linear Example Molecules.
From www.webassign.net
Chapter 6 Molecular Structure Linear Example Molecules A molecule with four electron groups about the central atom but only one electron group bonded to another atom is linear because there are only. Examples of molecules with linear geometry are carbon dioxide (co 2), beryllium chloride (becl 2), and nitric oxide (no). The atoms are arranged in a straight line, and the angle between the bonds, or bond.. Linear Example Molecules.
From www.slideserve.com
PPT The determination of point groups of molecules PowerPoint Linear Example Molecules Examples of molecules with linear geometry are carbon dioxide (co 2), beryllium chloride (becl 2), and nitric oxide (no). The atoms are arranged in a straight line, and the angle between the bonds is 180 °. The atoms are arranged in a straight line, and the angle between the bonds, or bond. It specifies the geometry shaped by a central. Linear Example Molecules.
From chem.libretexts.org
9.7 The Shapes of Molecules Chemistry LibreTexts Linear Example Molecules It refers to the geometry shaped by a central atom surrounded by two other atoms. If a molecule contains only two atoms, those two atoms are in a straight line and thus form a linear molecule. Understanding the molecular structure of a. The atoms are arranged in a straight line, and the angle between the bonds is 180 °. It. Linear Example Molecules.