Heat Entropy Equation . According to the equation, when the entropy decreases and enthalpy increases the free energy change, δg, is positive and not spontaneous,. We know from equation \(\ref{eq2}\) that the entropy change for any reversible process is the heat transferred (in joules) divided by the temperature at which the process occurs. As an example, suppose we mix equal masses of water originally at two different temperatures, say 20.0º c and 40.0º c. If the system absorbs heat—that is, with q > 0 q > 0 —the entropy of the system increases. The equation for the change in entropy, δ s δ s, is δ s = q t , δ s = q t , where q is the heat that transfers energy during a process, and t is the. By the clausius definition, if an amount of heat q flows into a large heat reservoir at temperature t above absolute zero, then the entropy increase is δs = q/t. Entropy is a measure of disorder. As an example, suppose a gas is kept at a constant temperature of 300 k while it absorbs 10 j of heat in. Its entropy increases because heat transfer occurs into it. The result is water at an intermediate temperature of 30.0º c. As an example, suppose a gas is kept at a constant.
from www.engineeringstream.com
As an example, suppose a gas is kept at a constant. As an example, suppose a gas is kept at a constant temperature of 300 k while it absorbs 10 j of heat in. Its entropy increases because heat transfer occurs into it. According to the equation, when the entropy decreases and enthalpy increases the free energy change, δg, is positive and not spontaneous,. The equation for the change in entropy, δ s δ s, is δ s = q t , δ s = q t , where q is the heat that transfers energy during a process, and t is the. The result is water at an intermediate temperature of 30.0º c. As an example, suppose we mix equal masses of water originally at two different temperatures, say 20.0º c and 40.0º c. If the system absorbs heat—that is, with q > 0 q > 0 —the entropy of the system increases. We know from equation \(\ref{eq2}\) that the entropy change for any reversible process is the heat transferred (in joules) divided by the temperature at which the process occurs. Entropy is a measure of disorder.
LAW OF THERMODYNAMICS Engineering Stream
Heat Entropy Equation If the system absorbs heat—that is, with q > 0 q > 0 —the entropy of the system increases. If the system absorbs heat—that is, with q > 0 q > 0 —the entropy of the system increases. Entropy is a measure of disorder. We know from equation \(\ref{eq2}\) that the entropy change for any reversible process is the heat transferred (in joules) divided by the temperature at which the process occurs. The result is water at an intermediate temperature of 30.0º c. As an example, suppose a gas is kept at a constant. Its entropy increases because heat transfer occurs into it. According to the equation, when the entropy decreases and enthalpy increases the free energy change, δg, is positive and not spontaneous,. As an example, suppose we mix equal masses of water originally at two different temperatures, say 20.0º c and 40.0º c. The equation for the change in entropy, δ s δ s, is δ s = q t , δ s = q t , where q is the heat that transfers energy during a process, and t is the. By the clausius definition, if an amount of heat q flows into a large heat reservoir at temperature t above absolute zero, then the entropy increase is δs = q/t. As an example, suppose a gas is kept at a constant temperature of 300 k while it absorbs 10 j of heat in.
From www.youtube.com
Thermodynamics 1 C2 L10 First law of thermodynamics Energy Heat Entropy Equation According to the equation, when the entropy decreases and enthalpy increases the free energy change, δg, is positive and not spontaneous,. The result is water at an intermediate temperature of 30.0º c. If the system absorbs heat—that is, with q > 0 q > 0 —the entropy of the system increases. Entropy is a measure of disorder. As an example,. Heat Entropy Equation.
From guidelibunveracity.z21.web.core.windows.net
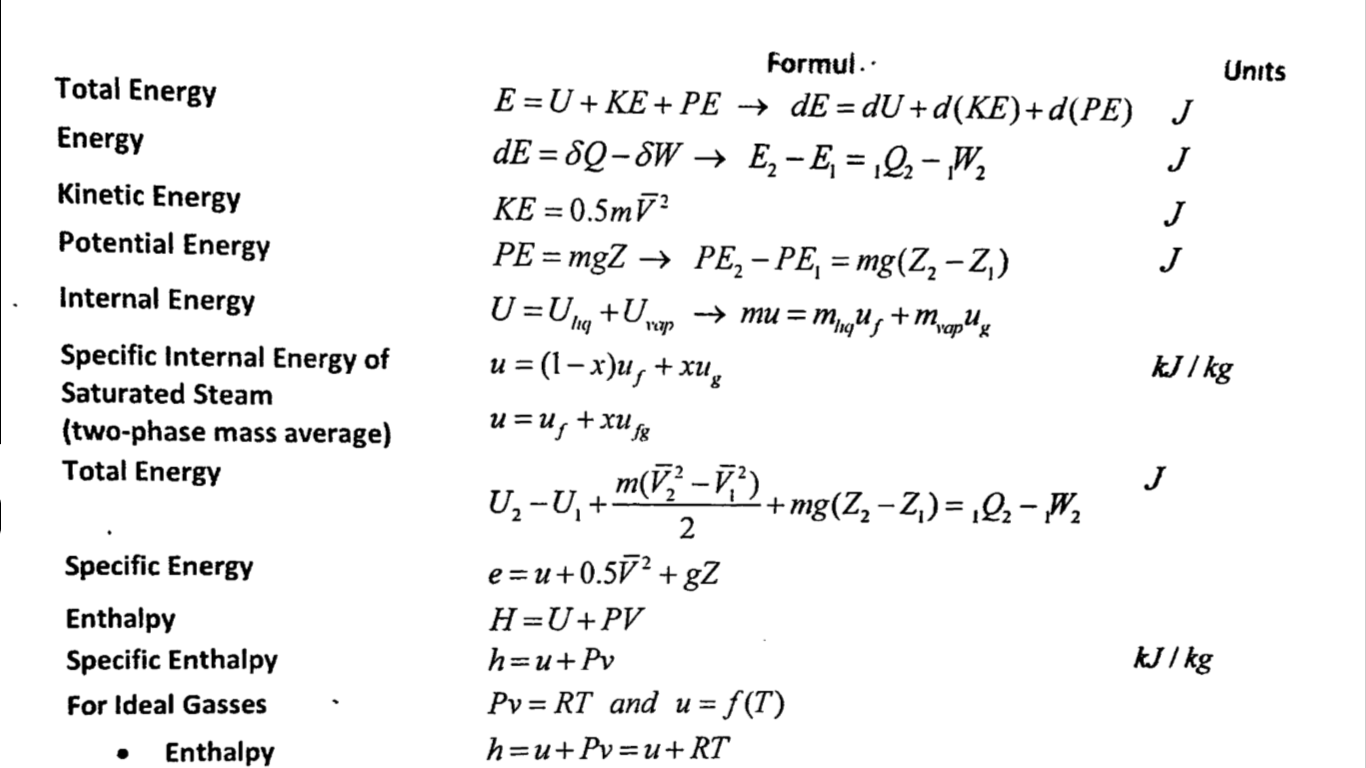
Entropy And Temp Relation Heat Entropy Equation We know from equation \(\ref{eq2}\) that the entropy change for any reversible process is the heat transferred (in joules) divided by the temperature at which the process occurs. As an example, suppose we mix equal masses of water originally at two different temperatures, say 20.0º c and 40.0º c. As an example, suppose a gas is kept at a constant. Heat Entropy Equation.
From classnotes.org.in
Enthalpies Of Reaction Chemistry, Class 11, Thermodynamics Heat Entropy Equation According to the equation, when the entropy decreases and enthalpy increases the free energy change, δg, is positive and not spontaneous,. Its entropy increases because heat transfer occurs into it. The equation for the change in entropy, δ s δ s, is δ s = q t , δ s = q t , where q is the heat that. Heat Entropy Equation.
From www.tessshebaylo.com
Equation For Heat Energy Tessshebaylo Heat Entropy Equation As an example, suppose a gas is kept at a constant temperature of 300 k while it absorbs 10 j of heat in. The result is water at an intermediate temperature of 30.0º c. If the system absorbs heat—that is, with q > 0 q > 0 —the entropy of the system increases. The equation for the change in entropy,. Heat Entropy Equation.
From www.slideserve.com
PPT Find the change in entropy when 87.3 g of water vapor condense Heat Entropy Equation As an example, suppose a gas is kept at a constant temperature of 300 k while it absorbs 10 j of heat in. Entropy is a measure of disorder. The equation for the change in entropy, δ s δ s, is δ s = q t , δ s = q t , where q is the heat that transfers. Heat Entropy Equation.
From www.chemicalslearning.com
Second Law of Thermodynamics in Terms of Entropy Heat Entropy Equation As an example, suppose a gas is kept at a constant. As an example, suppose a gas is kept at a constant temperature of 300 k while it absorbs 10 j of heat in. If the system absorbs heat—that is, with q > 0 q > 0 —the entropy of the system increases. By the clausius definition, if an amount. Heat Entropy Equation.
From www.tessshebaylo.com
Equation For Heat Energy Tessshebaylo Heat Entropy Equation If the system absorbs heat—that is, with q > 0 q > 0 —the entropy of the system increases. As an example, suppose a gas is kept at a constant. The equation for the change in entropy, δ s δ s, is δ s = q t , δ s = q t , where q is the heat that. Heat Entropy Equation.
From www.slideserve.com
PPT Calculating Entropy Change PowerPoint Presentation, free download Heat Entropy Equation By the clausius definition, if an amount of heat q flows into a large heat reservoir at temperature t above absolute zero, then the entropy increase is δs = q/t. We know from equation \(\ref{eq2}\) that the entropy change for any reversible process is the heat transferred (in joules) divided by the temperature at which the process occurs. Its entropy. Heat Entropy Equation.
From www.slideserve.com
PPT Chapter 8 ENTROPY PowerPoint Presentation, free download ID1794001 Heat Entropy Equation If the system absorbs heat—that is, with q > 0 q > 0 —the entropy of the system increases. The equation for the change in entropy, δ s δ s, is δ s = q t , δ s = q t , where q is the heat that transfers energy during a process, and t is the. As an. Heat Entropy Equation.
From www.slideserve.com
PPT Entropy Change by Heat Transfer PowerPoint Presentation, free Heat Entropy Equation As an example, suppose a gas is kept at a constant. As an example, suppose we mix equal masses of water originally at two different temperatures, say 20.0º c and 40.0º c. As an example, suppose a gas is kept at a constant temperature of 300 k while it absorbs 10 j of heat in. By the clausius definition, if. Heat Entropy Equation.
From www.linkedin.com
Is specific heat the same as entropy? Why both have the same SI unit kJ Heat Entropy Equation We know from equation \(\ref{eq2}\) that the entropy change for any reversible process is the heat transferred (in joules) divided by the temperature at which the process occurs. Entropy is a measure of disorder. The result is water at an intermediate temperature of 30.0º c. Its entropy increases because heat transfer occurs into it. By the clausius definition, if an. Heat Entropy Equation.
From www.youtube.com
Entropy generation associated with heat transfer YouTube Heat Entropy Equation If the system absorbs heat—that is, with q > 0 q > 0 —the entropy of the system increases. By the clausius definition, if an amount of heat q flows into a large heat reservoir at temperature t above absolute zero, then the entropy increase is δs = q/t. According to the equation, when the entropy decreases and enthalpy increases. Heat Entropy Equation.
From sciencenotes.org
What Is Entropy? Definition and Examples Heat Entropy Equation As an example, suppose a gas is kept at a constant temperature of 300 k while it absorbs 10 j of heat in. If the system absorbs heat—that is, with q > 0 q > 0 —the entropy of the system increases. The result is water at an intermediate temperature of 30.0º c. Entropy is a measure of disorder. By. Heat Entropy Equation.
From www.thesciencehive.co.uk
Enthalpy and Entropy* — the science sauce Heat Entropy Equation If the system absorbs heat—that is, with q > 0 q > 0 —the entropy of the system increases. By the clausius definition, if an amount of heat q flows into a large heat reservoir at temperature t above absolute zero, then the entropy increase is δs = q/t. Its entropy increases because heat transfer occurs into it. The result. Heat Entropy Equation.
From surfguppy.com
Example of Enthalpy Change Calculation Propane Combustion Heat Entropy Equation As an example, suppose a gas is kept at a constant temperature of 300 k while it absorbs 10 j of heat in. Entropy is a measure of disorder. As an example, suppose a gas is kept at a constant. As an example, suppose we mix equal masses of water originally at two different temperatures, say 20.0º c and 40.0º. Heat Entropy Equation.
From www.slideserve.com
PPT Energy, Enthalpy, and Thermochemistry PowerPoint Presentation Heat Entropy Equation According to the equation, when the entropy decreases and enthalpy increases the free energy change, δg, is positive and not spontaneous,. Entropy is a measure of disorder. Its entropy increases because heat transfer occurs into it. As an example, suppose we mix equal masses of water originally at two different temperatures, say 20.0º c and 40.0º c. If the system. Heat Entropy Equation.
From www.numerade.com
SOLVED The heat capacity at constant pressure (Cp) is the partial Heat Entropy Equation As an example, suppose we mix equal masses of water originally at two different temperatures, say 20.0º c and 40.0º c. As an example, suppose a gas is kept at a constant temperature of 300 k while it absorbs 10 j of heat in. As an example, suppose a gas is kept at a constant. Its entropy increases because heat. Heat Entropy Equation.
From studylib.net
Heat Equation Heat Entropy Equation By the clausius definition, if an amount of heat q flows into a large heat reservoir at temperature t above absolute zero, then the entropy increase is δs = q/t. As an example, suppose a gas is kept at a constant temperature of 300 k while it absorbs 10 j of heat in. We know from equation \(\ref{eq2}\) that the. Heat Entropy Equation.
From www.scribd.com
Thermodynamic Formulas Entropy Enthalpy Heat Entropy Equation As an example, suppose we mix equal masses of water originally at two different temperatures, say 20.0º c and 40.0º c. Entropy is a measure of disorder. As an example, suppose a gas is kept at a constant. Its entropy increases because heat transfer occurs into it. If the system absorbs heat—that is, with q > 0 q > 0. Heat Entropy Equation.
From www.youtube.com
Calculate Reaction Change in Enthalpy, Entropy and Free Energy YouTube Heat Entropy Equation According to the equation, when the entropy decreases and enthalpy increases the free energy change, δg, is positive and not spontaneous,. As an example, suppose a gas is kept at a constant. The equation for the change in entropy, δ s δ s, is δ s = q t , δ s = q t , where q is the. Heat Entropy Equation.
From www.youtube.com
Change in ENTHALPY Using Specific Heat at Constant Pressure in 3 Heat Entropy Equation By the clausius definition, if an amount of heat q flows into a large heat reservoir at temperature t above absolute zero, then the entropy increase is δs = q/t. The equation for the change in entropy, δ s δ s, is δ s = q t , δ s = q t , where q is the heat that. Heat Entropy Equation.
From www.engineeringstream.com
LAW OF THERMODYNAMICS Engineering Stream Heat Entropy Equation By the clausius definition, if an amount of heat q flows into a large heat reservoir at temperature t above absolute zero, then the entropy increase is δs = q/t. As an example, suppose a gas is kept at a constant. If the system absorbs heat—that is, with q > 0 q > 0 —the entropy of the system increases.. Heat Entropy Equation.
From wirelistattrahent.z13.web.core.windows.net
Enthalpy And Entropy Equation Heat Entropy Equation Entropy is a measure of disorder. As an example, suppose a gas is kept at a constant temperature of 300 k while it absorbs 10 j of heat in. As an example, suppose a gas is kept at a constant. The equation for the change in entropy, δ s δ s, is δ s = q t , δ s. Heat Entropy Equation.
From www.engineeringstream.com
LAW OF THERMODYNAMICS Engineering Stream Heat Entropy Equation As an example, suppose a gas is kept at a constant. The equation for the change in entropy, δ s δ s, is δ s = q t , δ s = q t , where q is the heat that transfers energy during a process, and t is the. As an example, suppose we mix equal masses of water. Heat Entropy Equation.
From www.youtube.com
REFERENCE ENTROPY and Specific Heats in 12 Minutes! YouTube Heat Entropy Equation Entropy is a measure of disorder. As an example, suppose a gas is kept at a constant. By the clausius definition, if an amount of heat q flows into a large heat reservoir at temperature t above absolute zero, then the entropy increase is δs = q/t. If the system absorbs heat—that is, with q > 0 q > 0. Heat Entropy Equation.
From elife-news.blogspot.com
ELifes Exercise Thermodynamics (enthalpy, heat of melting, heat Heat Entropy Equation By the clausius definition, if an amount of heat q flows into a large heat reservoir at temperature t above absolute zero, then the entropy increase is δs = q/t. According to the equation, when the entropy decreases and enthalpy increases the free energy change, δg, is positive and not spontaneous,. Entropy is a measure of disorder. The equation for. Heat Entropy Equation.
From www.slideserve.com
PPT Calculating Entropy Change PowerPoint Presentation, free download Heat Entropy Equation The result is water at an intermediate temperature of 30.0º c. According to the equation, when the entropy decreases and enthalpy increases the free energy change, δg, is positive and not spontaneous,. The equation for the change in entropy, δ s δ s, is δ s = q t , δ s = q t , where q is the. Heat Entropy Equation.
From www.youtube.com
Mechanical Engineering Thermodynamics Lec 10, pt 1 of 2 Entropy Heat Entropy Equation As an example, suppose a gas is kept at a constant temperature of 300 k while it absorbs 10 j of heat in. As an example, suppose a gas is kept at a constant. If the system absorbs heat—that is, with q > 0 q > 0 —the entropy of the system increases. According to the equation, when the entropy. Heat Entropy Equation.
From www.youtube.com
Change in Entropy Using Specific Heats in 3 Minutes! YouTube Heat Entropy Equation The equation for the change in entropy, δ s δ s, is δ s = q t , δ s = q t , where q is the heat that transfers energy during a process, and t is the. By the clausius definition, if an amount of heat q flows into a large heat reservoir at temperature t above absolute. Heat Entropy Equation.
From studylib.net
2.5(a) Enthalpy Heat Entropy Equation Entropy is a measure of disorder. As an example, suppose a gas is kept at a constant. By the clausius definition, if an amount of heat q flows into a large heat reservoir at temperature t above absolute zero, then the entropy increase is δs = q/t. As an example, suppose a gas is kept at a constant temperature of. Heat Entropy Equation.
From www.youtube.com
The First Law of Thermodynamics Internal Energy, Heat, and Work YouTube Heat Entropy Equation If the system absorbs heat—that is, with q > 0 q > 0 —the entropy of the system increases. As an example, suppose a gas is kept at a constant. By the clausius definition, if an amount of heat q flows into a large heat reservoir at temperature t above absolute zero, then the entropy increase is δs = q/t.. Heat Entropy Equation.
From slidetodoc.com
Sensible Heat and Enthalpy Calculations constant A Enthalpy Heat Entropy Equation If the system absorbs heat—that is, with q > 0 q > 0 —the entropy of the system increases. As an example, suppose a gas is kept at a constant temperature of 300 k while it absorbs 10 j of heat in. Entropy is a measure of disorder. The equation for the change in entropy, δ s δ s, is. Heat Entropy Equation.
From www.chemistrylearner.com
First Law of Thermodynamics Statement, Equation, & Examples Heat Entropy Equation If the system absorbs heat—that is, with q > 0 q > 0 —the entropy of the system increases. Its entropy increases because heat transfer occurs into it. According to the equation, when the entropy decreases and enthalpy increases the free energy change, δg, is positive and not spontaneous,. By the clausius definition, if an amount of heat q flows. Heat Entropy Equation.
From diagramlibraryisadore.z21.web.core.windows.net
Entropy Enthalpy Temperature Equation Heat Entropy Equation As an example, suppose a gas is kept at a constant temperature of 300 k while it absorbs 10 j of heat in. According to the equation, when the entropy decreases and enthalpy increases the free energy change, δg, is positive and not spontaneous,. The equation for the change in entropy, δ s δ s, is δ s = q. Heat Entropy Equation.
From studylib.net
Thermodynamics Formulas Heat Entropy Equation As an example, suppose we mix equal masses of water originally at two different temperatures, say 20.0º c and 40.0º c. By the clausius definition, if an amount of heat q flows into a large heat reservoir at temperature t above absolute zero, then the entropy increase is δs = q/t. The equation for the change in entropy, δ s. Heat Entropy Equation.