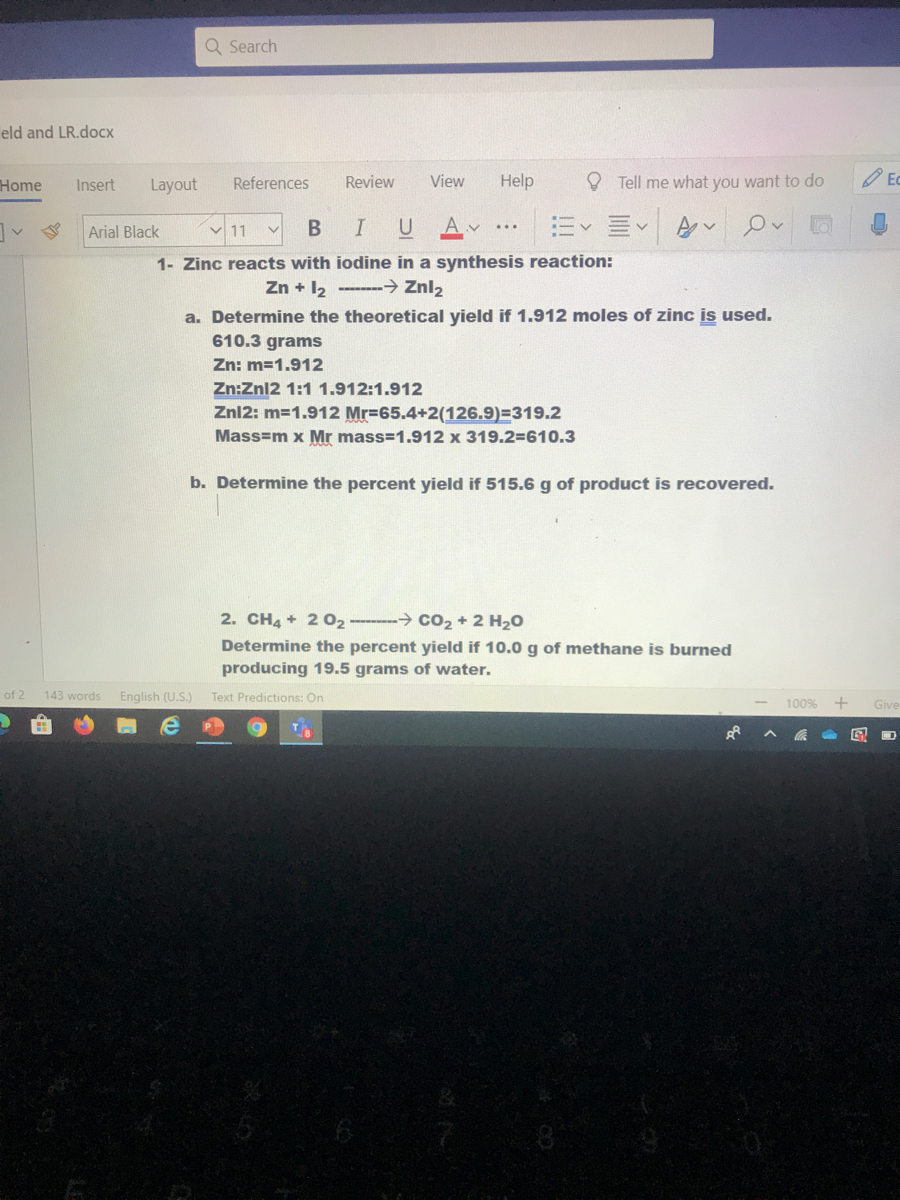
Zinc Iodine Grams . We assume you are converting between moles zinc iodide and. Zn + i 2 → zni 2 How many moles zinc iodide in 1 grams? Zinc powder is added to a solution of iodine in ethanol. An exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. To calculate molar mass of a chemical compound enter its formula and click 'compute'. 1 grams zinc iodide = 0.0031329406338453 mole using the molecular weight calculator and the molar mass of zni2. Zinc iodide appears as a white crystalline solid. This free moles to grams calculator can instantly convert moles to grams and determine how many atoms/molecules are present in these grams.
from www.bartleby.com
To calculate molar mass of a chemical compound enter its formula and click 'compute'. Zinc iodide appears as a white crystalline solid. 1 grams zinc iodide = 0.0031329406338453 mole using the molecular weight calculator and the molar mass of zni2. We assume you are converting between moles zinc iodide and. Zinc powder is added to a solution of iodine in ethanol. How many moles zinc iodide in 1 grams? Zn + i 2 → zni 2 This free moles to grams calculator can instantly convert moles to grams and determine how many atoms/molecules are present in these grams. An exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent.
Answered 1 Zinc reacts with iodine in a… bartleby
Zinc Iodine Grams This free moles to grams calculator can instantly convert moles to grams and determine how many atoms/molecules are present in these grams. Zinc powder is added to a solution of iodine in ethanol. An exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. This free moles to grams calculator can instantly convert moles to grams and determine how many atoms/molecules are present in these grams. We assume you are converting between moles zinc iodide and. Zn + i 2 → zni 2 How many moles zinc iodide in 1 grams? 1 grams zinc iodide = 0.0031329406338453 mole using the molecular weight calculator and the molar mass of zni2. To calculate molar mass of a chemical compound enter its formula and click 'compute'. Zinc iodide appears as a white crystalline solid.
From www.shopclues.com
Buy labogens zinc iodide 25gm Online ₹1450 from ShopClues Zinc Iodine Grams Zinc iodide appears as a white crystalline solid. To calculate molar mass of a chemical compound enter its formula and click 'compute'. How many moles zinc iodide in 1 grams? An exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. Zinc powder is added to a solution of iodine in ethanol. We assume you. Zinc Iodine Grams.
From www.grainger.com
SPECTRUM Zinc Iodide, Purified, 25g 39H156Z107325GM04 Grainger Zinc Iodine Grams To calculate molar mass of a chemical compound enter its formula and click 'compute'. 1 grams zinc iodide = 0.0031329406338453 mole using the molecular weight calculator and the molar mass of zni2. An exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. We assume you are converting between moles zinc iodide and. Zinc iodide. Zinc Iodine Grams.
From www.chegg.com
Solved Finding the Empirical Formula of Zinc Iodide Report Zinc Iodine Grams This free moles to grams calculator can instantly convert moles to grams and determine how many atoms/molecules are present in these grams. We assume you are converting between moles zinc iodide and. How many moles zinc iodide in 1 grams? Zn + i 2 → zni 2 Zinc iodide appears as a white crystalline solid. Zinc powder is added to. Zinc Iodine Grams.
From www.grainger.com
SPECTRUM Zinc Iodide, Purified, 500g 39H157Z1073500GM10 Grainger Zinc Iodine Grams How many moles zinc iodide in 1 grams? Zn + i 2 → zni 2 An exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. This free moles to grams calculator can instantly convert moles to grams and determine how many atoms/molecules are present in these grams. 1 grams zinc iodide = 0.0031329406338453 mole. Zinc Iodine Grams.
From www.shutterstock.com
Zinc Iodide Zni2 Molecule Simple Molecular Stock Vector (Royalty Free Zinc Iodine Grams How many moles zinc iodide in 1 grams? An exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. Zinc iodide appears as a white crystalline solid. To calculate molar mass of a chemical compound enter its formula and click 'compute'. Zinc powder is added to a solution of iodine in ethanol. Zn + i. Zinc Iodine Grams.
From www.chegg.com
Solved Finding the Empirical Formula of Zinc Iodide Report Zinc Iodine Grams This free moles to grams calculator can instantly convert moles to grams and determine how many atoms/molecules are present in these grams. Zinc iodide appears as a white crystalline solid. An exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. To calculate molar mass of a chemical compound enter its formula and click 'compute'.. Zinc Iodine Grams.
From www.chegg.com
Solved Finding the Empirical Formula of Zinc Iodide Report Zinc Iodine Grams Zn + i 2 → zni 2 To calculate molar mass of a chemical compound enter its formula and click 'compute'. An exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. 1 grams zinc iodide = 0.0031329406338453 mole using the molecular weight calculator and the molar mass of zni2. We assume you are converting. Zinc Iodine Grams.
From www.alamy.com
ZnI2 Zinc iodide. Chemical compound. CAS number 10139476 Stock Zinc Iodine Grams How many moles zinc iodide in 1 grams? We assume you are converting between moles zinc iodide and. Zn + i 2 → zni 2 This free moles to grams calculator can instantly convert moles to grams and determine how many atoms/molecules are present in these grams. Zinc powder is added to a solution of iodine in ethanol. An exothermic. Zinc Iodine Grams.
From www.researchgate.net
a) Schematic reactions in zinc‐iodine batteries Zn reversibly reacts Zinc Iodine Grams How many moles zinc iodide in 1 grams? An exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. Zinc powder is added to a solution of iodine in ethanol. Zinc iodide appears as a white crystalline solid. Zn + i 2 → zni 2 We assume you are converting between moles zinc iodide and.. Zinc Iodine Grams.
From www.researchgate.net
(PDF) Synthesis and of Zinc Iodide Model Reactions for Zinc Iodine Grams Zinc iodide appears as a white crystalline solid. Zinc powder is added to a solution of iodine in ethanol. Zn + i 2 → zni 2 We assume you are converting between moles zinc iodide and. How many moles zinc iodide in 1 grams? An exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent.. Zinc Iodine Grams.
From www.walmart.com
Zinc Iodine Zinc Iodine Grams Zinc powder is added to a solution of iodine in ethanol. This free moles to grams calculator can instantly convert moles to grams and determine how many atoms/molecules are present in these grams. Zinc iodide appears as a white crystalline solid. How many moles zinc iodide in 1 grams? We assume you are converting between moles zinc iodide and. To. Zinc Iodine Grams.
From www.toppr.com
In the reaction between zinc and iodine , zinc iodide is formed . What Zinc Iodine Grams We assume you are converting between moles zinc iodide and. An exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. 1 grams zinc iodide = 0.0031329406338453 mole using the molecular weight calculator and the molar mass of zni2. This free moles to grams calculator can instantly convert moles to grams and determine how many. Zinc Iodine Grams.
From fphoto.photoshelter.com
science chemistry exothermic reaction iodine zinc Fundamental Zinc Iodine Grams An exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. Zn + i 2 → zni 2 This free moles to grams calculator can instantly convert moles to grams and determine how many atoms/molecules are present in these grams. Zinc iodide appears as a white crystalline solid. 1 grams zinc iodide = 0.0031329406338453 mole. Zinc Iodine Grams.
From www.bartleby.com
Answered 1 Zinc reacts with iodine in a… bartleby Zinc Iodine Grams Zn + i 2 → zni 2 How many moles zinc iodide in 1 grams? 1 grams zinc iodide = 0.0031329406338453 mole using the molecular weight calculator and the molar mass of zni2. An exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. This free moles to grams calculator can instantly convert moles to. Zinc Iodine Grams.
From www.youtube.com
How to Write the Formula for Zinc iodide YouTube Zinc Iodine Grams An exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. Zn + i 2 → zni 2 To calculate molar mass of a chemical compound enter its formula and click 'compute'. This free moles to grams calculator can instantly convert moles to grams and determine how many atoms/molecules are present in these grams. Zinc. Zinc Iodine Grams.
From www.researchgate.net
Stable Solid‐State ZincIodine Batteries Enabled by an ZnPS3 Zinc Iodine Grams 1 grams zinc iodide = 0.0031329406338453 mole using the molecular weight calculator and the molar mass of zni2. How many moles zinc iodide in 1 grams? An exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. We assume you are converting between moles zinc iodide and. Zinc powder is added to a solution of. Zinc Iodine Grams.
From www.coursehero.com
[Solved] Data analysis reaction of zinc and iodine (limiting reactant Zinc Iodine Grams 1 grams zinc iodide = 0.0031329406338453 mole using the molecular weight calculator and the molar mass of zni2. Zn + i 2 → zni 2 Zinc iodide appears as a white crystalline solid. How many moles zinc iodide in 1 grams? To calculate molar mass of a chemical compound enter its formula and click 'compute'. This free moles to grams. Zinc Iodine Grams.
From www.dreamstime.com
Zinc Iodide or Zn2 Iodide, Powder. Chemical Compound of Zinc and Iodine Zinc Iodine Grams How many moles zinc iodide in 1 grams? Zn + i 2 → zni 2 To calculate molar mass of a chemical compound enter its formula and click 'compute'. Zinc iodide appears as a white crystalline solid. Zinc powder is added to a solution of iodine in ethanol. 1 grams zinc iodide = 0.0031329406338453 mole using the molecular weight calculator. Zinc Iodine Grams.
From www.transtutors.com
(Get Answer) Finding the Empirical Formula of Zinc Iodide Report Zinc Iodine Grams An exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. How many moles zinc iodide in 1 grams? Zinc iodide appears as a white crystalline solid. Zn + i 2 → zni 2 Zinc powder is added to a solution of iodine in ethanol. This free moles to grams calculator can instantly convert moles. Zinc Iodine Grams.
From www.youtube.com
Gram Stain Preparation of Gram's Iodine YouTube Zinc Iodine Grams Zinc iodide appears as a white crystalline solid. This free moles to grams calculator can instantly convert moles to grams and determine how many atoms/molecules are present in these grams. Zinc powder is added to a solution of iodine in ethanol. Zn + i 2 → zni 2 1 grams zinc iodide = 0.0031329406338453 mole using the molecular weight calculator. Zinc Iodine Grams.
From www.coursehero.com
[Solved] 1.2 grams Zn in moles and 2.3 grams of iodine to moles Zinc Iodine Grams Zinc powder is added to a solution of iodine in ethanol. Zn + i 2 → zni 2 1 grams zinc iodide = 0.0031329406338453 mole using the molecular weight calculator and the molar mass of zni2. We assume you are converting between moles zinc iodide and. Zinc iodide appears as a white crystalline solid. To calculate molar mass of a. Zinc Iodine Grams.
From www.grainger.com
SPECTRUM Zinc Iodide, Purified, 100g 39H155Z1073100GM06 Grainger Zinc Iodine Grams This free moles to grams calculator can instantly convert moles to grams and determine how many atoms/molecules are present in these grams. Zinc iodide appears as a white crystalline solid. 1 grams zinc iodide = 0.0031329406338453 mole using the molecular weight calculator and the molar mass of zni2. Zinc powder is added to a solution of iodine in ethanol. To. Zinc Iodine Grams.
From www.pw.live
Zinc Iodide Formula, Structure, Properties And Uses Zinc Iodine Grams Zinc powder is added to a solution of iodine in ethanol. We assume you are converting between moles zinc iodide and. To calculate molar mass of a chemical compound enter its formula and click 'compute'. An exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. Zinc iodide appears as a white crystalline solid. Zn. Zinc Iodine Grams.
From www.numerade.com
SOLVED Zinc reacts with iodine in a synthesis reaction. Using a Zinc Iodine Grams An exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. 1 grams zinc iodide = 0.0031329406338453 mole using the molecular weight calculator and the molar mass of zni2. We assume you are converting between moles zinc iodide and. Zinc powder is added to a solution of iodine in ethanol. Zn + i 2 →. Zinc Iodine Grams.
From www.amazon.com
ALDON Innovating Science Gram's Iodine Solution, 1 fl oz (30mL) The Zinc Iodine Grams Zinc powder is added to a solution of iodine in ethanol. Zinc iodide appears as a white crystalline solid. To calculate molar mass of a chemical compound enter its formula and click 'compute'. 1 grams zinc iodide = 0.0031329406338453 mole using the molecular weight calculator and the molar mass of zni2. An exothermic redox reaction occurs, forming zinc iodide, which. Zinc Iodine Grams.
From www.coursehero.com
[Solved] Data analysis reaction of zinc and iodine (limiting reactant Zinc Iodine Grams To calculate molar mass of a chemical compound enter its formula and click 'compute'. Zinc iodide appears as a white crystalline solid. An exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. We assume you are converting between moles zinc iodide and. Zinc powder is added to a solution of iodine in ethanol. 1. Zinc Iodine Grams.
From www.coursehero.com
[Solved] Data analysis reaction of zinc and iodine (limiting reactant Zinc Iodine Grams Zn + i 2 → zni 2 Zinc iodide appears as a white crystalline solid. This free moles to grams calculator can instantly convert moles to grams and determine how many atoms/molecules are present in these grams. An exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. Zinc powder is added to a solution. Zinc Iodine Grams.
From www.deltamicroscopies.com
Gram's Iodine Solution Delta Microscopies Zinc Iodine Grams Zinc powder is added to a solution of iodine in ethanol. 1 grams zinc iodide = 0.0031329406338453 mole using the molecular weight calculator and the molar mass of zni2. Zinc iodide appears as a white crystalline solid. We assume you are converting between moles zinc iodide and. This free moles to grams calculator can instantly convert moles to grams and. Zinc Iodine Grams.
From www.sciencephoto.com
Zinc reacts with iodine Stock Image C044/0436 Science Photo Library Zinc Iodine Grams How many moles zinc iodide in 1 grams? 1 grams zinc iodide = 0.0031329406338453 mole using the molecular weight calculator and the molar mass of zni2. This free moles to grams calculator can instantly convert moles to grams and determine how many atoms/molecules are present in these grams. An exothermic redox reaction occurs, forming zinc iodide, which can be obtained. Zinc Iodine Grams.
From www.coursehero.com
Calculating the Empirical Formula of a Compound ZincIodine Course Hero Zinc Iodine Grams Zinc iodide appears as a white crystalline solid. This free moles to grams calculator can instantly convert moles to grams and determine how many atoms/molecules are present in these grams. We assume you are converting between moles zinc iodide and. How many moles zinc iodide in 1 grams? To calculate molar mass of a chemical compound enter its formula and. Zinc Iodine Grams.
From www.researchgate.net
Zinc, selenium, and iodine contents in 100 g of lamb depending on the Zinc Iodine Grams 1 grams zinc iodide = 0.0031329406338453 mole using the molecular weight calculator and the molar mass of zni2. To calculate molar mass of a chemical compound enter its formula and click 'compute'. How many moles zinc iodide in 1 grams? This free moles to grams calculator can instantly convert moles to grams and determine how many atoms/molecules are present in. Zinc Iodine Grams.
From www.researchgate.net
a) Schematic illustration for the open structure of zinc‐iodine (Zn‐I2 Zinc Iodine Grams Zinc powder is added to a solution of iodine in ethanol. 1 grams zinc iodide = 0.0031329406338453 mole using the molecular weight calculator and the molar mass of zni2. To calculate molar mass of a chemical compound enter its formula and click 'compute'. How many moles zinc iodide in 1 grams? An exothermic redox reaction occurs, forming zinc iodide, which. Zinc Iodine Grams.
From www.indiamart.com
IODINE SOLUTION (GRAM'S) at Rs 70/litre in Faridabad ID 2851089937430 Zinc Iodine Grams Zn + i 2 → zni 2 An exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. To calculate molar mass of a chemical compound enter its formula and click 'compute'. This free moles to grams calculator can instantly convert moles to grams and determine how many atoms/molecules are present in these grams. Zinc. Zinc Iodine Grams.
From www.coursehero.com
[Solved] Data analysis reaction of zinc and iodine (limiting reactant Zinc Iodine Grams Zinc powder is added to a solution of iodine in ethanol. This free moles to grams calculator can instantly convert moles to grams and determine how many atoms/molecules are present in these grams. Zinc iodide appears as a white crystalline solid. How many moles zinc iodide in 1 grams? Zn + i 2 → zni 2 1 grams zinc iodide. Zinc Iodine Grams.
From www.algeria.ubuy.com
Selenium 300mcg Zinc 50mg Iodine 500mcg L Tyrosine Ubuy Algeria Zinc Iodine Grams Zinc powder is added to a solution of iodine in ethanol. How many moles zinc iodide in 1 grams? We assume you are converting between moles zinc iodide and. Zinc iodide appears as a white crystalline solid. An exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. To calculate molar mass of a chemical. Zinc Iodine Grams.