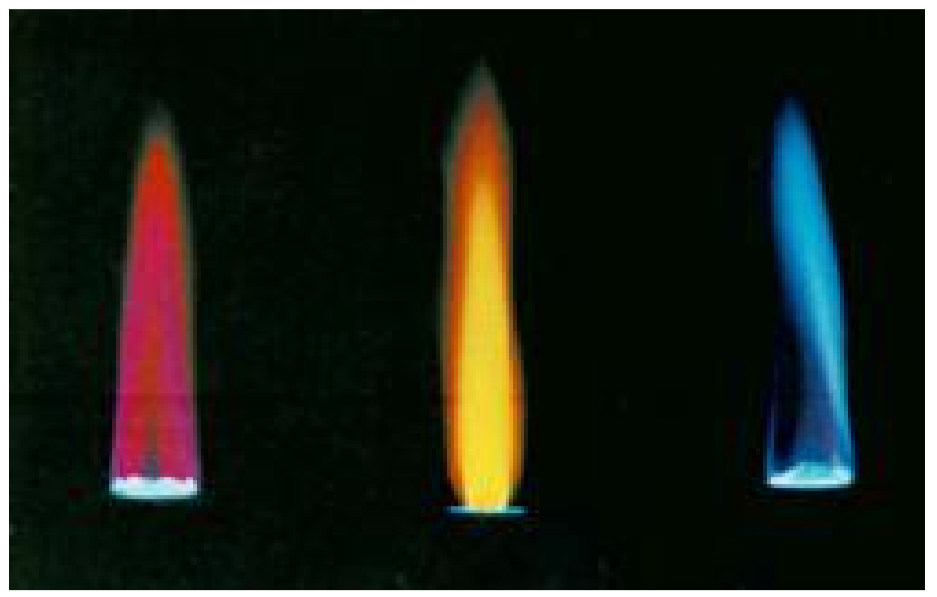
Virtual Lab Flame Test & Spectroscopy . Perform flame tests of metal cations in order to observe their characteristic colors, perform. Then using a spectroscope, match the bright line. in this virtual lab you will: the objectives of this lab are to: this activity combines experimental flame tests with a simulation exploring emissions from discharge. 2.use a flame test to. Use a flame test to determine which ion salt produces the red color. in this virtual investigation you will burn a small amount of various metal salt solutions and record the colors of the flames. 1.observe the bright line spectra (emission spectra) for various elements. Flame tests and identification of an unknown metal) to. learn flame test procedure and how to detect the presence of metal ions in their salts either, powder form or solutions in the flame test virtual lab. on my teacher website, find the link for the spectroscopy virtual lab (part 1:
from chem.libretexts.org
in this virtual lab you will: 1.observe the bright line spectra (emission spectra) for various elements. in this virtual investigation you will burn a small amount of various metal salt solutions and record the colors of the flames. learn flame test procedure and how to detect the presence of metal ions in their salts either, powder form or solutions in the flame test virtual lab. this activity combines experimental flame tests with a simulation exploring emissions from discharge. 2.use a flame test to. the objectives of this lab are to: Then using a spectroscope, match the bright line. on my teacher website, find the link for the spectroscopy virtual lab (part 1: Use a flame test to determine which ion salt produces the red color.
5 Flame Tests and Atomic Spectra (Experiment) Chemistry LibreTexts
Virtual Lab Flame Test & Spectroscopy in this virtual lab you will: Then using a spectroscope, match the bright line. in this virtual investigation you will burn a small amount of various metal salt solutions and record the colors of the flames. this activity combines experimental flame tests with a simulation exploring emissions from discharge. in this virtual lab you will: on my teacher website, find the link for the spectroscopy virtual lab (part 1: Flame tests and identification of an unknown metal) to. 2.use a flame test to. Perform flame tests of metal cations in order to observe their characteristic colors, perform. 1.observe the bright line spectra (emission spectra) for various elements. the objectives of this lab are to: Use a flame test to determine which ion salt produces the red color. learn flame test procedure and how to detect the presence of metal ions in their salts either, powder form or solutions in the flame test virtual lab.
From ar.inspiredpencil.com
Flame Test Lab Virtual Lab Flame Test & Spectroscopy this activity combines experimental flame tests with a simulation exploring emissions from discharge. in this virtual investigation you will burn a small amount of various metal salt solutions and record the colors of the flames. in this virtual lab you will: 1.observe the bright line spectra (emission spectra) for various elements. on my teacher website, find. Virtual Lab Flame Test & Spectroscopy.
From stemfinity.com
Flame Tests and Emission Spectroscopy Experiment Kit LabAids Virtual Lab Flame Test & Spectroscopy Then using a spectroscope, match the bright line. on my teacher website, find the link for the spectroscopy virtual lab (part 1: in this virtual investigation you will burn a small amount of various metal salt solutions and record the colors of the flames. the objectives of this lab are to: in this virtual lab you. Virtual Lab Flame Test & Spectroscopy.
From www.youtube.com
Flame Atomic Absorption Spectroscopy Demonstration YouTube Virtual Lab Flame Test & Spectroscopy Perform flame tests of metal cations in order to observe their characteristic colors, perform. this activity combines experimental flame tests with a simulation exploring emissions from discharge. learn flame test procedure and how to detect the presence of metal ions in their salts either, powder form or solutions in the flame test virtual lab. Then using a spectroscope,. Virtual Lab Flame Test & Spectroscopy.
From www.studypool.com
SOLUTION Flame test amp spectroscopy virtual lab compress Studypool Virtual Lab Flame Test & Spectroscopy Use a flame test to determine which ion salt produces the red color. 1.observe the bright line spectra (emission spectra) for various elements. the objectives of this lab are to: learn flame test procedure and how to detect the presence of metal ions in their salts either, powder form or solutions in the flame test virtual lab. Perform. Virtual Lab Flame Test & Spectroscopy.
From www.blendspace.com
Flame Test Virtual Lab Lessons Blendspace Virtual Lab Flame Test & Spectroscopy on my teacher website, find the link for the spectroscopy virtual lab (part 1: in this virtual investigation you will burn a small amount of various metal salt solutions and record the colors of the flames. Then using a spectroscope, match the bright line. 1.observe the bright line spectra (emission spectra) for various elements. in this virtual. Virtual Lab Flame Test & Spectroscopy.
From www.slideserve.com
PPT Flame Test Virtual Lab PowerPoint Presentation, free download Virtual Lab Flame Test & Spectroscopy in this virtual investigation you will burn a small amount of various metal salt solutions and record the colors of the flames. Perform flame tests of metal cations in order to observe their characteristic colors, perform. in this virtual lab you will: Then using a spectroscope, match the bright line. learn flame test procedure and how to. Virtual Lab Flame Test & Spectroscopy.
From cupsoguepictures.com
😀 Flame tests of metal cations answers. Properties of Cations Flame Virtual Lab Flame Test & Spectroscopy Flame tests and identification of an unknown metal) to. Perform flame tests of metal cations in order to observe their characteristic colors, perform. the objectives of this lab are to: in this virtual investigation you will burn a small amount of various metal salt solutions and record the colors of the flames. learn flame test procedure and. Virtual Lab Flame Test & Spectroscopy.
From slidetodoc.com
Lab 10 Flame Tests Atomic Spectra Date Purpose Virtual Lab Flame Test & Spectroscopy in this virtual lab you will: in this virtual investigation you will burn a small amount of various metal salt solutions and record the colors of the flames. Perform flame tests of metal cations in order to observe their characteristic colors, perform. 2.use a flame test to. Then using a spectroscope, match the bright line. this activity. Virtual Lab Flame Test & Spectroscopy.
From praxilabs.com
As Featured In Virtual Lab Flame Test & Spectroscopy Then using a spectroscope, match the bright line. learn flame test procedure and how to detect the presence of metal ions in their salts either, powder form or solutions in the flame test virtual lab. in this virtual investigation you will burn a small amount of various metal salt solutions and record the colors of the flames. Flame. Virtual Lab Flame Test & Spectroscopy.
From elchoroukhost.net
Periodic Table Virtual Lab Elcho Table Virtual Lab Flame Test & Spectroscopy in this virtual lab you will: Flame tests and identification of an unknown metal) to. this activity combines experimental flame tests with a simulation exploring emissions from discharge. learn flame test procedure and how to detect the presence of metal ions in their salts either, powder form or solutions in the flame test virtual lab. Then using. Virtual Lab Flame Test & Spectroscopy.
From www.youtube.com
Flame Test Lab YouTube Virtual Lab Flame Test & Spectroscopy learn flame test procedure and how to detect the presence of metal ions in their salts either, powder form or solutions in the flame test virtual lab. 2.use a flame test to. 1.observe the bright line spectra (emission spectra) for various elements. on my teacher website, find the link for the spectroscopy virtual lab (part 1: Perform flame. Virtual Lab Flame Test & Spectroscopy.
From www.youtube.com
Virtual Lab Flame Tests YouTube Virtual Lab Flame Test & Spectroscopy 1.observe the bright line spectra (emission spectra) for various elements. Flame tests and identification of an unknown metal) to. learn flame test procedure and how to detect the presence of metal ions in their salts either, powder form or solutions in the flame test virtual lab. on my teacher website, find the link for the spectroscopy virtual lab. Virtual Lab Flame Test & Spectroscopy.
From exoviqakk.blob.core.windows.net
Emission Spectra And Flame Tests Virtual Lab at Anthony Cornell blog Virtual Lab Flame Test & Spectroscopy in this virtual lab you will: on my teacher website, find the link for the spectroscopy virtual lab (part 1: this activity combines experimental flame tests with a simulation exploring emissions from discharge. 1.observe the bright line spectra (emission spectra) for various elements. the objectives of this lab are to: Perform flame tests of metal cations. Virtual Lab Flame Test & Spectroscopy.
From www.animalia-life.club
Flame Test Lab Results Virtual Lab Flame Test & Spectroscopy the objectives of this lab are to: learn flame test procedure and how to detect the presence of metal ions in their salts either, powder form or solutions in the flame test virtual lab. Flame tests and identification of an unknown metal) to. Perform flame tests of metal cations in order to observe their characteristic colors, perform. . Virtual Lab Flame Test & Spectroscopy.
From studylib.net
Emission Spectrum Lab and Flame Test Discussion Questions Virtual Lab Flame Test & Spectroscopy the objectives of this lab are to: Perform flame tests of metal cations in order to observe their characteristic colors, perform. in this virtual lab you will: learn flame test procedure and how to detect the presence of metal ions in their salts either, powder form or solutions in the flame test virtual lab. in this. Virtual Lab Flame Test & Spectroscopy.
From studylib.net
Flame Test Virtual Lab Virtual Lab Flame Test & Spectroscopy Flame tests and identification of an unknown metal) to. 2.use a flame test to. Perform flame tests of metal cations in order to observe their characteristic colors, perform. in this virtual investigation you will burn a small amount of various metal salt solutions and record the colors of the flames. in this virtual lab you will: Then using. Virtual Lab Flame Test & Spectroscopy.
From exoviqakk.blob.core.windows.net
Emission Spectra And Flame Tests Virtual Lab at Anthony Cornell blog Virtual Lab Flame Test & Spectroscopy the objectives of this lab are to: in this virtual lab you will: Flame tests and identification of an unknown metal) to. this activity combines experimental flame tests with a simulation exploring emissions from discharge. Use a flame test to determine which ion salt produces the red color. in this virtual investigation you will burn a. Virtual Lab Flame Test & Spectroscopy.
From www.scribd.com
Flame Test & Spectroscopy Virtual Lab PDF Emission Spectrum Virtual Lab Flame Test & Spectroscopy on my teacher website, find the link for the spectroscopy virtual lab (part 1: Then using a spectroscope, match the bright line. 2.use a flame test to. the objectives of this lab are to: in this virtual lab you will: Use a flame test to determine which ion salt produces the red color. learn flame test. Virtual Lab Flame Test & Spectroscopy.
From www.studypool.com
SOLUTION Flame test virtual lab Studypool Virtual Lab Flame Test & Spectroscopy in this virtual lab you will: 1.observe the bright line spectra (emission spectra) for various elements. learn flame test procedure and how to detect the presence of metal ions in their salts either, powder form or solutions in the flame test virtual lab. Perform flame tests of metal cations in order to observe their characteristic colors, perform. . Virtual Lab Flame Test & Spectroscopy.
From www.mrpalermo.com
Virtual Lab Flame Test & Spectroscopy Mr. Palermo's Flipped Virtual Lab Flame Test & Spectroscopy on my teacher website, find the link for the spectroscopy virtual lab (part 1: Flame tests and identification of an unknown metal) to. this activity combines experimental flame tests with a simulation exploring emissions from discharge. Then using a spectroscope, match the bright line. 1.observe the bright line spectra (emission spectra) for various elements. in this virtual. Virtual Lab Flame Test & Spectroscopy.
From www.slideserve.com
PPT Flame Test Virtual Lab PowerPoint Presentation, free download Virtual Lab Flame Test & Spectroscopy Perform flame tests of metal cations in order to observe their characteristic colors, perform. learn flame test procedure and how to detect the presence of metal ions in their salts either, powder form or solutions in the flame test virtual lab. 1.observe the bright line spectra (emission spectra) for various elements. Use a flame test to determine which ion. Virtual Lab Flame Test & Spectroscopy.
From www.youtube.com
Flame Test Lab YouTube Virtual Lab Flame Test & Spectroscopy this activity combines experimental flame tests with a simulation exploring emissions from discharge. 2.use a flame test to. Flame tests and identification of an unknown metal) to. on my teacher website, find the link for the spectroscopy virtual lab (part 1: Use a flame test to determine which ion salt produces the red color. learn flame test. Virtual Lab Flame Test & Spectroscopy.
From physicsopenlab.org
Flame Test PhysicsOpenLab Virtual Lab Flame Test & Spectroscopy Flame tests and identification of an unknown metal) to. the objectives of this lab are to: Then using a spectroscope, match the bright line. in this virtual lab you will: learn flame test procedure and how to detect the presence of metal ions in their salts either, powder form or solutions in the flame test virtual lab.. Virtual Lab Flame Test & Spectroscopy.
From www.animalia-life.club
Flame Test Lab Results Virtual Lab Flame Test & Spectroscopy Then using a spectroscope, match the bright line. learn flame test procedure and how to detect the presence of metal ions in their salts either, powder form or solutions in the flame test virtual lab. 1.observe the bright line spectra (emission spectra) for various elements. Use a flame test to determine which ion salt produces the red color. Perform. Virtual Lab Flame Test & Spectroscopy.
From studylib.net
Flame Test Lab Virtual Lab Flame Test & Spectroscopy Flame tests and identification of an unknown metal) to. Use a flame test to determine which ion salt produces the red color. Perform flame tests of metal cations in order to observe their characteristic colors, perform. in this virtual investigation you will burn a small amount of various metal salt solutions and record the colors of the flames. Then. Virtual Lab Flame Test & Spectroscopy.
From studymind.co.uk
Flame Emission Spectroscopy (GCSE Chemistry) Study Mind Virtual Lab Flame Test & Spectroscopy Flame tests and identification of an unknown metal) to. in this virtual investigation you will burn a small amount of various metal salt solutions and record the colors of the flames. Then using a spectroscope, match the bright line. this activity combines experimental flame tests with a simulation exploring emissions from discharge. 2.use a flame test to. Perform. Virtual Lab Flame Test & Spectroscopy.
From knowledge.carolina.com
Flame Test Colors and BrightLine Emission Spectra for Metal Chlorides Virtual Lab Flame Test & Spectroscopy 1.observe the bright line spectra (emission spectra) for various elements. this activity combines experimental flame tests with a simulation exploring emissions from discharge. 2.use a flame test to. in this virtual investigation you will burn a small amount of various metal salt solutions and record the colors of the flames. learn flame test procedure and how to. Virtual Lab Flame Test & Spectroscopy.
From www.youtube.com
Flame photometry/Flame Emission Spectroscopy (FES)/Atomic emission Virtual Lab Flame Test & Spectroscopy in this virtual lab you will: on my teacher website, find the link for the spectroscopy virtual lab (part 1: in this virtual investigation you will burn a small amount of various metal salt solutions and record the colors of the flames. Then using a spectroscope, match the bright line. 2.use a flame test to. Use a. Virtual Lab Flame Test & Spectroscopy.
From www.youtube.com
Measuring flame temperature with thermocouple virtual laboratory Virtual Lab Flame Test & Spectroscopy the objectives of this lab are to: in this virtual lab you will: Use a flame test to determine which ion salt produces the red color. 1.observe the bright line spectra (emission spectra) for various elements. Perform flame tests of metal cations in order to observe their characteristic colors, perform. on my teacher website, find the link. Virtual Lab Flame Test & Spectroscopy.
From www.scribd.com
Lab Flame Test Handout Chem Emission Spectrum Scientific Techniques Virtual Lab Flame Test & Spectroscopy 1.observe the bright line spectra (emission spectra) for various elements. this activity combines experimental flame tests with a simulation exploring emissions from discharge. in this virtual investigation you will burn a small amount of various metal salt solutions and record the colors of the flames. Flame tests and identification of an unknown metal) to. the objectives of. Virtual Lab Flame Test & Spectroscopy.
From www.chegg.com
Solved Flame Test & Spectroscopy Background Electrons are Virtual Lab Flame Test & Spectroscopy the objectives of this lab are to: 2.use a flame test to. 1.observe the bright line spectra (emission spectra) for various elements. this activity combines experimental flame tests with a simulation exploring emissions from discharge. Flame tests and identification of an unknown metal) to. on my teacher website, find the link for the spectroscopy virtual lab (part. Virtual Lab Flame Test & Spectroscopy.
From chem.libretexts.org
5 Flame Tests and Atomic Spectra (Experiment) Chemistry LibreTexts Virtual Lab Flame Test & Spectroscopy Use a flame test to determine which ion salt produces the red color. Then using a spectroscope, match the bright line. in this virtual investigation you will burn a small amount of various metal salt solutions and record the colors of the flames. this activity combines experimental flame tests with a simulation exploring emissions from discharge. 2.use a. Virtual Lab Flame Test & Spectroscopy.
From www.studocu.com
Copy of Flame Test Virtual Lab Flame Test Virtual Lab Document Virtual Lab Flame Test & Spectroscopy Then using a spectroscope, match the bright line. Flame tests and identification of an unknown metal) to. in this virtual lab you will: 2.use a flame test to. Use a flame test to determine which ion salt produces the red color. Perform flame tests of metal cations in order to observe their characteristic colors, perform. in this virtual. Virtual Lab Flame Test & Spectroscopy.
From www.youtube.com
Flame Test Lab YouTube Virtual Lab Flame Test & Spectroscopy Flame tests and identification of an unknown metal) to. Use a flame test to determine which ion salt produces the red color. Perform flame tests of metal cations in order to observe their characteristic colors, perform. 1.observe the bright line spectra (emission spectra) for various elements. in this virtual lab you will: Then using a spectroscope, match the bright. Virtual Lab Flame Test & Spectroscopy.
From exoviqakk.blob.core.windows.net
Emission Spectra And Flame Tests Virtual Lab at Anthony Cornell blog Virtual Lab Flame Test & Spectroscopy this activity combines experimental flame tests with a simulation exploring emissions from discharge. Use a flame test to determine which ion salt produces the red color. 1.observe the bright line spectra (emission spectra) for various elements. Flame tests and identification of an unknown metal) to. learn flame test procedure and how to detect the presence of metal ions. Virtual Lab Flame Test & Spectroscopy.