What Is The Definition Of Drug Product . A substance intended for use in the diagnosis, cure,. A drug product is deemed to be a listed drug on the date of approval for the nda or anda for that drug product. (4) drug product means a finished dosage form, for example, tablet, capsule, solution, etc., that contains an active drug ingredient generally, but. Repack or repackage means the act of taking a finished drug product or unfinished drug from the container in which it was placed. A substance recognized by an official pharmacopoeia or formulary. (1) a product comprised of two or more regulated components, i.e., drug/device, biologic/device, drug/biologic, or drug/device/biologic, that are. A drug substance, also known as an active pharmaceutical ingredient (api), is the specific chemical compound or molecule within a drug product that provides the.
from pharmapproach.com
Repack or repackage means the act of taking a finished drug product or unfinished drug from the container in which it was placed. A substance intended for use in the diagnosis, cure,. A drug substance, also known as an active pharmaceutical ingredient (api), is the specific chemical compound or molecule within a drug product that provides the. A substance recognized by an official pharmacopoeia or formulary. (1) a product comprised of two or more regulated components, i.e., drug/device, biologic/device, drug/biologic, or drug/device/biologic, that are. A drug product is deemed to be a listed drug on the date of approval for the nda or anda for that drug product. (4) drug product means a finished dosage form, for example, tablet, capsule, solution, etc., that contains an active drug ingredient generally, but.
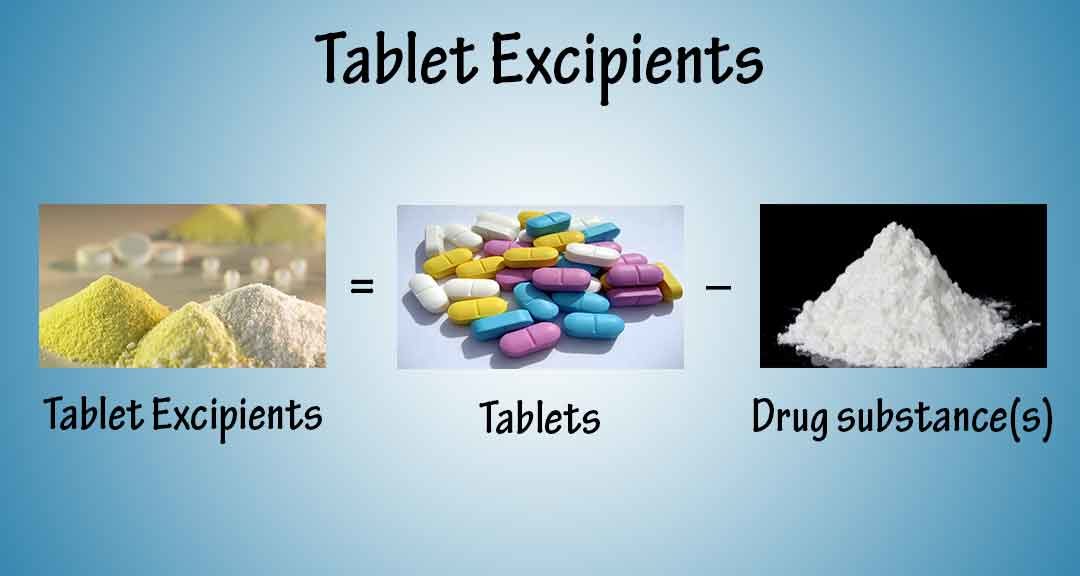
Excipients Used In the Manufacture of Tablets
What Is The Definition Of Drug Product A drug substance, also known as an active pharmaceutical ingredient (api), is the specific chemical compound or molecule within a drug product that provides the. Repack or repackage means the act of taking a finished drug product or unfinished drug from the container in which it was placed. (4) drug product means a finished dosage form, for example, tablet, capsule, solution, etc., that contains an active drug ingredient generally, but. A substance intended for use in the diagnosis, cure,. A substance recognized by an official pharmacopoeia or formulary. (1) a product comprised of two or more regulated components, i.e., drug/device, biologic/device, drug/biologic, or drug/device/biologic, that are. A drug substance, also known as an active pharmaceutical ingredient (api), is the specific chemical compound or molecule within a drug product that provides the. A drug product is deemed to be a listed drug on the date of approval for the nda or anda for that drug product.
From chamowassociates.com
Drug Product Critical Quality Attributes Chamow and Associates What Is The Definition Of Drug Product A drug product is deemed to be a listed drug on the date of approval for the nda or anda for that drug product. A drug substance, also known as an active pharmaceutical ingredient (api), is the specific chemical compound or molecule within a drug product that provides the. Repack or repackage means the act of taking a finished drug. What Is The Definition Of Drug Product.
From www.researchgate.net
(PDF) Process validation of drug products What Is The Definition Of Drug Product (1) a product comprised of two or more regulated components, i.e., drug/device, biologic/device, drug/biologic, or drug/device/biologic, that are. Repack or repackage means the act of taking a finished drug product or unfinished drug from the container in which it was placed. A drug substance, also known as an active pharmaceutical ingredient (api), is the specific chemical compound or molecule within. What Is The Definition Of Drug Product.
From blogs.ubc.ca
Generic vs. BrandName Drugs What’s the difference? Communicating What Is The Definition Of Drug Product A drug product is deemed to be a listed drug on the date of approval for the nda or anda for that drug product. (4) drug product means a finished dosage form, for example, tablet, capsule, solution, etc., that contains an active drug ingredient generally, but. A substance intended for use in the diagnosis, cure,. (1) a product comprised of. What Is The Definition Of Drug Product.
From easconsultinggroup.com
Drugs EAS Consulting Group What Is The Definition Of Drug Product A drug product is deemed to be a listed drug on the date of approval for the nda or anda for that drug product. Repack or repackage means the act of taking a finished drug product or unfinished drug from the container in which it was placed. A substance intended for use in the diagnosis, cure,. A substance recognized by. What Is The Definition Of Drug Product.
From www.aislac.org
The Difference Between Drug Substance and Drug Product AISLAC What Is The Definition Of Drug Product A substance recognized by an official pharmacopoeia or formulary. (1) a product comprised of two or more regulated components, i.e., drug/device, biologic/device, drug/biologic, or drug/device/biologic, that are. A drug substance, also known as an active pharmaceutical ingredient (api), is the specific chemical compound or molecule within a drug product that provides the. (4) drug product means a finished dosage form,. What Is The Definition Of Drug Product.
From emergencydrug.com
True Difference Between Generic and Brand Drug Emergency Drug What Is The Definition Of Drug Product A substance recognized by an official pharmacopoeia or formulary. (4) drug product means a finished dosage form, for example, tablet, capsule, solution, etc., that contains an active drug ingredient generally, but. A drug product is deemed to be a listed drug on the date of approval for the nda or anda for that drug product. Repack or repackage means the. What Is The Definition Of Drug Product.
From www.drugwatch.com
How to Read OvertheCounter and Prescription Drug Labels What Is The Definition Of Drug Product Repack or repackage means the act of taking a finished drug product or unfinished drug from the container in which it was placed. A drug substance, also known as an active pharmaceutical ingredient (api), is the specific chemical compound or molecule within a drug product that provides the. A drug product is deemed to be a listed drug on the. What Is The Definition Of Drug Product.
From www.slideserve.com
PPT Product Development Case Study Overview PowerPoint Presentation What Is The Definition Of Drug Product (1) a product comprised of two or more regulated components, i.e., drug/device, biologic/device, drug/biologic, or drug/device/biologic, that are. A substance intended for use in the diagnosis, cure,. Repack or repackage means the act of taking a finished drug product or unfinished drug from the container in which it was placed. (4) drug product means a finished dosage form, for example,. What Is The Definition Of Drug Product.
From www.fda.gov
OTC Drug Facts Label FDA What Is The Definition Of Drug Product A drug product is deemed to be a listed drug on the date of approval for the nda or anda for that drug product. (1) a product comprised of two or more regulated components, i.e., drug/device, biologic/device, drug/biologic, or drug/device/biologic, that are. (4) drug product means a finished dosage form, for example, tablet, capsule, solution, etc., that contains an active. What Is The Definition Of Drug Product.
From www.pharma-excipients.ch
Definition of Pharmaceutical Excipients pharma excipients What Is The Definition Of Drug Product A drug product is deemed to be a listed drug on the date of approval for the nda or anda for that drug product. A drug substance, also known as an active pharmaceutical ingredient (api), is the specific chemical compound or molecule within a drug product that provides the. A substance intended for use in the diagnosis, cure,. A substance. What Is The Definition Of Drug Product.
From www.slideserve.com
PPT FDA’s Critical Path to Medical Product Development Opportunities What Is The Definition Of Drug Product (1) a product comprised of two or more regulated components, i.e., drug/device, biologic/device, drug/biologic, or drug/device/biologic, that are. A drug product is deemed to be a listed drug on the date of approval for the nda or anda for that drug product. (4) drug product means a finished dosage form, for example, tablet, capsule, solution, etc., that contains an active. What Is The Definition Of Drug Product.
From pharmacampus.in
What is an API or Active Pharmaceutical Ingredient? > PharmaCampus What Is The Definition Of Drug Product Repack or repackage means the act of taking a finished drug product or unfinished drug from the container in which it was placed. (4) drug product means a finished dosage form, for example, tablet, capsule, solution, etc., that contains an active drug ingredient generally, but. A substance intended for use in the diagnosis, cure,. A drug substance, also known as. What Is The Definition Of Drug Product.
From lubrizolcdmo.com
Key Takeaways From the FDA Complex Generic Drug Product Development What Is The Definition Of Drug Product A drug product is deemed to be a listed drug on the date of approval for the nda or anda for that drug product. Repack or repackage means the act of taking a finished drug product or unfinished drug from the container in which it was placed. (4) drug product means a finished dosage form, for example, tablet, capsule, solution,. What Is The Definition Of Drug Product.
From libraryguides.ccbcmd.edu
Pharmacology Drug & Pharmacology Research Guides at Community What Is The Definition Of Drug Product A drug substance, also known as an active pharmaceutical ingredient (api), is the specific chemical compound or molecule within a drug product that provides the. Repack or repackage means the act of taking a finished drug product or unfinished drug from the container in which it was placed. (1) a product comprised of two or more regulated components, i.e., drug/device,. What Is The Definition Of Drug Product.
From pharmapproach.com
Excipients Used In the Manufacture of Tablets What Is The Definition Of Drug Product A substance intended for use in the diagnosis, cure,. (4) drug product means a finished dosage form, for example, tablet, capsule, solution, etc., that contains an active drug ingredient generally, but. A drug product is deemed to be a listed drug on the date of approval for the nda or anda for that drug product. A substance recognized by an. What Is The Definition Of Drug Product.
From www.slideserve.com
PPT Pharmaceutical Nomenclature Issues and challenges PowerPoint What Is The Definition Of Drug Product Repack or repackage means the act of taking a finished drug product or unfinished drug from the container in which it was placed. A substance recognized by an official pharmacopoeia or formulary. A substance intended for use in the diagnosis, cure,. A drug substance, also known as an active pharmaceutical ingredient (api), is the specific chemical compound or molecule within. What Is The Definition Of Drug Product.
From www.youtube.com
Drug Formulation & Delivery with Dr. Robert Ternik YouTube What Is The Definition Of Drug Product A substance recognized by an official pharmacopoeia or formulary. (1) a product comprised of two or more regulated components, i.e., drug/device, biologic/device, drug/biologic, or drug/device/biologic, that are. A drug substance, also known as an active pharmaceutical ingredient (api), is the specific chemical compound or molecule within a drug product that provides the. A drug product is deemed to be a. What Is The Definition Of Drug Product.
From www.slideserve.com
PPT Drug and Product Labeling PowerPoint Presentation, free download What Is The Definition Of Drug Product A drug substance, also known as an active pharmaceutical ingredient (api), is the specific chemical compound or molecule within a drug product that provides the. (1) a product comprised of two or more regulated components, i.e., drug/device, biologic/device, drug/biologic, or drug/device/biologic, that are. Repack or repackage means the act of taking a finished drug product or unfinished drug from the. What Is The Definition Of Drug Product.
From www.pharmaexcipients.com
Oxidation of Drugs during Drug Product Development Problems and What Is The Definition Of Drug Product (4) drug product means a finished dosage form, for example, tablet, capsule, solution, etc., that contains an active drug ingredient generally, but. A substance intended for use in the diagnosis, cure,. A drug product is deemed to be a listed drug on the date of approval for the nda or anda for that drug product. (1) a product comprised of. What Is The Definition Of Drug Product.
From www.totalpharmaceuticaltopics.com
Drug Substance and Drug Product Manufacturing Flow Discovery to Delivery What Is The Definition Of Drug Product (1) a product comprised of two or more regulated components, i.e., drug/device, biologic/device, drug/biologic, or drug/device/biologic, that are. A substance recognized by an official pharmacopoeia or formulary. A drug product is deemed to be a listed drug on the date of approval for the nda or anda for that drug product. (4) drug product means a finished dosage form, for. What Is The Definition Of Drug Product.
From marketbusinessnews.com
Generic definition and meaning Market Business News What Is The Definition Of Drug Product A substance intended for use in the diagnosis, cure,. A drug product is deemed to be a listed drug on the date of approval for the nda or anda for that drug product. A substance recognized by an official pharmacopoeia or formulary. (1) a product comprised of two or more regulated components, i.e., drug/device, biologic/device, drug/biologic, or drug/device/biologic, that are.. What Is The Definition Of Drug Product.
From www.slideserve.com
PPT Nature and Nomenclature of Drugs PowerPoint Presentation, free What Is The Definition Of Drug Product A substance intended for use in the diagnosis, cure,. (1) a product comprised of two or more regulated components, i.e., drug/device, biologic/device, drug/biologic, or drug/device/biologic, that are. A drug product is deemed to be a listed drug on the date of approval for the nda or anda for that drug product. A substance recognized by an official pharmacopoeia or formulary.. What Is The Definition Of Drug Product.
From slideplayer.com
Dorota Matecka, Ph.D. Office of Pharmaceutical Quality (OPQ), CDER What Is The Definition Of Drug Product (4) drug product means a finished dosage form, for example, tablet, capsule, solution, etc., that contains an active drug ingredient generally, but. A drug substance, also known as an active pharmaceutical ingredient (api), is the specific chemical compound or molecule within a drug product that provides the. (1) a product comprised of two or more regulated components, i.e., drug/device, biologic/device,. What Is The Definition Of Drug Product.
From www.canada.ca
Classification of products under the Food and Drugs Act (F&DA) Canada.ca What Is The Definition Of Drug Product A substance recognized by an official pharmacopoeia or formulary. (1) a product comprised of two or more regulated components, i.e., drug/device, biologic/device, drug/biologic, or drug/device/biologic, that are. A substance intended for use in the diagnosis, cure,. A drug substance, also known as an active pharmaceutical ingredient (api), is the specific chemical compound or molecule within a drug product that provides. What Is The Definition Of Drug Product.
From basicmedicalkey.com
Introduction to Pharmacology Basicmedical Key What Is The Definition Of Drug Product (1) a product comprised of two or more regulated components, i.e., drug/device, biologic/device, drug/biologic, or drug/device/biologic, that are. A substance recognized by an official pharmacopoeia or formulary. (4) drug product means a finished dosage form, for example, tablet, capsule, solution, etc., that contains an active drug ingredient generally, but. A substance intended for use in the diagnosis, cure,. Repack or. What Is The Definition Of Drug Product.
From www.slideserve.com
PPT Product Development Case Study Overview PowerPoint Presentation What Is The Definition Of Drug Product (4) drug product means a finished dosage form, for example, tablet, capsule, solution, etc., that contains an active drug ingredient generally, but. A drug substance, also known as an active pharmaceutical ingredient (api), is the specific chemical compound or molecule within a drug product that provides the. (1) a product comprised of two or more regulated components, i.e., drug/device, biologic/device,. What Is The Definition Of Drug Product.
From mavink.com
Parts Of Drug Label What Is The Definition Of Drug Product A substance recognized by an official pharmacopoeia or formulary. A drug substance, also known as an active pharmaceutical ingredient (api), is the specific chemical compound or molecule within a drug product that provides the. (4) drug product means a finished dosage form, for example, tablet, capsule, solution, etc., that contains an active drug ingredient generally, but. Repack or repackage means. What Is The Definition Of Drug Product.
From www.slideteam.net
Drug Substance Specification Pharmaceutical Development New Medicine What Is The Definition Of Drug Product A substance recognized by an official pharmacopoeia or formulary. A substance intended for use in the diagnosis, cure,. (4) drug product means a finished dosage form, for example, tablet, capsule, solution, etc., that contains an active drug ingredient generally, but. A drug product is deemed to be a listed drug on the date of approval for the nda or anda. What Is The Definition Of Drug Product.
From hubpages.com
What Are the Pharmaceutical Sources of Drugs? HubPages What Is The Definition Of Drug Product A substance recognized by an official pharmacopoeia or formulary. Repack or repackage means the act of taking a finished drug product or unfinished drug from the container in which it was placed. A drug substance, also known as an active pharmaceutical ingredient (api), is the specific chemical compound or molecule within a drug product that provides the. (1) a product. What Is The Definition Of Drug Product.
From www.slideserve.com
PPT Label of the Drug Product PowerPoint Presentation, free download What Is The Definition Of Drug Product (1) a product comprised of two or more regulated components, i.e., drug/device, biologic/device, drug/biologic, or drug/device/biologic, that are. A drug product is deemed to be a listed drug on the date of approval for the nda or anda for that drug product. Repack or repackage means the act of taking a finished drug product or unfinished drug from the container. What Is The Definition Of Drug Product.
From www.bioserendipity.com
Biologics and Small Molecule Drugs BioSerendipity What Is The Definition Of Drug Product Repack or repackage means the act of taking a finished drug product or unfinished drug from the container in which it was placed. A drug product is deemed to be a listed drug on the date of approval for the nda or anda for that drug product. A substance recognized by an official pharmacopoeia or formulary. (1) a product comprised. What Is The Definition Of Drug Product.
From www.slideserve.com
PPT the physical and chemical properties of the drug substance What Is The Definition Of Drug Product A drug substance, also known as an active pharmaceutical ingredient (api), is the specific chemical compound or molecule within a drug product that provides the. A drug product is deemed to be a listed drug on the date of approval for the nda or anda for that drug product. A substance intended for use in the diagnosis, cure,. (1) a. What Is The Definition Of Drug Product.
From deneyimbelgesi.blogspot.com
Fda drug products What Is The Definition Of Drug Product Repack or repackage means the act of taking a finished drug product or unfinished drug from the container in which it was placed. A substance recognized by an official pharmacopoeia or formulary. (4) drug product means a finished dosage form, for example, tablet, capsule, solution, etc., that contains an active drug ingredient generally, but. A drug product is deemed to. What Is The Definition Of Drug Product.
From www.youtube.com
BIOPHARMACEUTIC CONSIDERATIONS IN DRUG PRODUCT DESIGN AND IN VITRO DRUG What Is The Definition Of Drug Product A substance recognized by an official pharmacopoeia or formulary. (4) drug product means a finished dosage form, for example, tablet, capsule, solution, etc., that contains an active drug ingredient generally, but. Repack or repackage means the act of taking a finished drug product or unfinished drug from the container in which it was placed. (1) a product comprised of two. What Is The Definition Of Drug Product.
From hubpages.com
What Are the Pharmaceutical Sources of Drugs? HubPages What Is The Definition Of Drug Product Repack or repackage means the act of taking a finished drug product or unfinished drug from the container in which it was placed. A drug product is deemed to be a listed drug on the date of approval for the nda or anda for that drug product. A substance intended for use in the diagnosis, cure,. (1) a product comprised. What Is The Definition Of Drug Product.