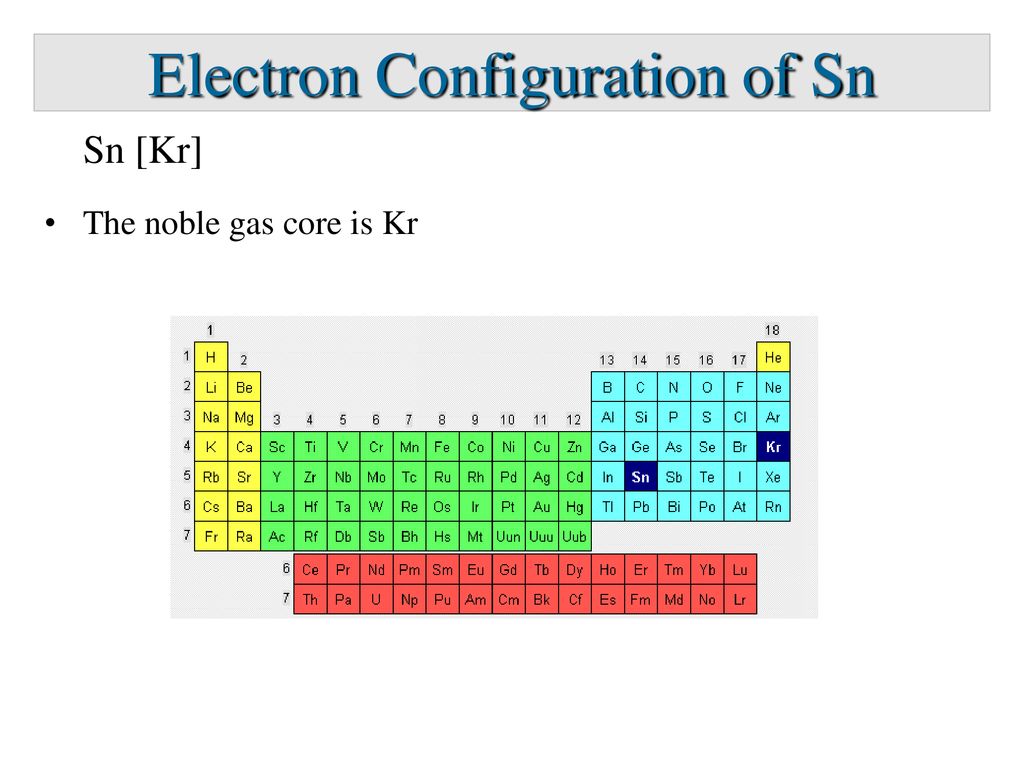
Tin 2 Electrons Lost Ion . It can sometimes lose two electrons to form the \(\ce{fe^{2+}}\) ion, while at other times it loses three electrons to form the \(\ce{fe^{3+}}\) ion. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e., usually eight valence electrons for the main group elements, called an octet. So, these two electrons will be lost from the last 5p orbital. Tin and lead, though members of. The tin electron configuration, represented as 5s 2 4d 10 5p 2 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2, illustrates the precise arrangement of electrons. Define the two types of ions. During bond formation, the tin atom donates. When a tin atom loses two electrons it exhibits a +2 charge.
from slideplayer.com
Define the two types of ions. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e., usually eight valence electrons for the main group elements, called an octet. So, these two electrons will be lost from the last 5p orbital. It can sometimes lose two electrons to form the \(\ce{fe^{2+}}\) ion, while at other times it loses three electrons to form the \(\ce{fe^{3+}}\) ion. When a tin atom loses two electrons it exhibits a +2 charge. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. Tin and lead, though members of. During bond formation, the tin atom donates. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when. The tin electron configuration, represented as 5s 2 4d 10 5p 2 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2, illustrates the precise arrangement of electrons.
ELECTRON CONFIGURATION. ppt download
Tin 2 Electrons Lost Ion Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. Define the two types of ions. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e., usually eight valence electrons for the main group elements, called an octet. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. When a tin atom loses two electrons it exhibits a +2 charge. During bond formation, the tin atom donates. So, these two electrons will be lost from the last 5p orbital. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when. Tin and lead, though members of. The tin electron configuration, represented as 5s 2 4d 10 5p 2 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2, illustrates the precise arrangement of electrons. It can sometimes lose two electrons to form the \(\ce{fe^{2+}}\) ion, while at other times it loses three electrons to form the \(\ce{fe^{3+}}\) ion.
From www.numerade.com
SOLVED Determine the number of electrons lost or gained when each atom Tin 2 Electrons Lost Ion During bond formation, the tin atom donates. So, these two electrons will be lost from the last 5p orbital. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when. The tin electron configuration, represented as 5s 2 4d 10 5p 2 or 1s. Tin 2 Electrons Lost Ion.
From valenceelectrons.com
How Many Protons, Neutrons and Electrons Does Tin Have? Tin 2 Electrons Lost Ion During bond formation, the tin atom donates. Tin and lead, though members of. The tin electron configuration, represented as 5s 2 4d 10 5p 2 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2, illustrates the precise arrangement of electrons. A cation (a positive ion) forms. Tin 2 Electrons Lost Ion.
From www.slideserve.com
PPT Structure & Properties of Matter PowerPoint Presentation ID5671688 Tin 2 Electrons Lost Ion So, these two electrons will be lost from the last 5p orbital. The tin electron configuration, represented as 5s 2 4d 10 5p 2 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2, illustrates the precise arrangement of electrons. Atoms tend to lose, gain, or share. Tin 2 Electrons Lost Ion.
From valenceelectrons.com
Electron Configuration for Tin and Tin ion(Sn2+, Sn4+) Tin 2 Electrons Lost Ion A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. It can sometimes lose two electrons to form the \(\ce{fe^{2+}}\) ion, while. Tin 2 Electrons Lost Ion.
From www.toppr.com
If three electrons are lost by a metal ion M3+, its final oxidation Tin 2 Electrons Lost Ion So, these two electrons will be lost from the last 5p orbital. The tin electron configuration, represented as 5s 2 4d 10 5p 2 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2, illustrates the precise arrangement of electrons. Tin and lead, though members of. Define. Tin 2 Electrons Lost Ion.
From spmchemistry.blog.onlinetuition.com.my
Formation of Ion SPM Chemistry Tin 2 Electrons Lost Ion It can sometimes lose two electrons to form the \(\ce{fe^{2+}}\) ion, while at other times it loses three electrons to form the \(\ce{fe^{3+}}\) ion. During bond formation, the tin atom donates. Define the two types of ions. When a tin atom loses two electrons it exhibits a +2 charge. A cation (a positive ion) forms when a neutral atom loses. Tin 2 Electrons Lost Ion.
From www.britannica.com
Tin Definition, Properties, Uses, & Facts Britannica Tin 2 Electrons Lost Ion Define the two types of ions. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. It can sometimes lose two electrons to form the \(\ce{fe^{2+}}\) ion, while at other times it loses three electrons to form the \(\ce{fe^{3+}}\) ion. The tin electron configuration, represented as 5s 2 4d. Tin 2 Electrons Lost Ion.
From www.slideserve.com
PPT Chemistry 120 PowerPoint Presentation, free download ID2089971 Tin 2 Electrons Lost Ion The tin electron configuration, represented as 5s 2 4d 10 5p 2 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2, illustrates the precise arrangement of electrons. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell,. Tin 2 Electrons Lost Ion.
From valenceelectrons.com
Tin(Sn) electron configuration and orbital diagram Tin 2 Electrons Lost Ion Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e., usually eight valence electrons for the main group elements, called an octet. Tin and lead, though members of. It can sometimes lose two electrons to form the \(\ce{fe^{2+}}\) ion, while at other times it loses three electrons. Tin 2 Electrons Lost Ion.
From periodictable.me
Tin Electron Configuration (Sn) with Orbital Diagram Tin 2 Electrons Lost Ion The tin electron configuration, represented as 5s 2 4d 10 5p 2 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2, illustrates the precise arrangement of electrons. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance. Tin 2 Electrons Lost Ion.
From sarai-kobrien.blogspot.com
Metals Tend to Lose Electrons to Positive Ions Tin 2 Electrons Lost Ion Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. So, these two electrons will be lost from the last 5p orbital. During bond formation, the tin atom donates. The tin electron configuration, represented as 5s 2 4d 10 5p 2 or 1s 2 2s 2 2p 6 3s. Tin 2 Electrons Lost Ion.
From www.schoolmykids.com
Tin (Sn) Element Information, Facts, Properties, Uses Periodic Tin 2 Electrons Lost Ion The tin electron configuration, represented as 5s 2 4d 10 5p 2 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2, illustrates the precise arrangement of electrons. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell,. Tin 2 Electrons Lost Ion.
From www.newtondesk.com
Tin Sn (Element 50) of Periodic Table Periodic Table FlashCards Tin 2 Electrons Lost Ion A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when. It can sometimes lose two electrons to form the \(\ce{fe^{2+}}\) ion, while at other times it loses three electrons to form the \(\ce{fe^{3+}}\) ion. During bond formation, the tin atom donates. Atoms that. Tin 2 Electrons Lost Ion.
From www.slideshare.net
Understanding The Chemistry Of Atoms To Ions Tin 2 Electrons Lost Ion So, these two electrons will be lost from the last 5p orbital. Define the two types of ions. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. The tin electron configuration, represented as 5s 2 4d 10 5p 2 or 1s 2 2s 2 2p 6 3s 2. Tin 2 Electrons Lost Ion.
From www.vectorstock.com
Symbol and electron diagram for Tin Royalty Free Vector Tin 2 Electrons Lost Ion So, these two electrons will be lost from the last 5p orbital. When a tin atom loses two electrons it exhibits a +2 charge. The tin electron configuration, represented as 5s 2 4d 10 5p 2 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2, illustrates. Tin 2 Electrons Lost Ion.
From general.chemistrysteps.com
Electron Configurations of Ions Chemistry Steps Tin 2 Electrons Lost Ion It can sometimes lose two electrons to form the \(\ce{fe^{2+}}\) ion, while at other times it loses three electrons to form the \(\ce{fe^{3+}}\) ion. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e., usually eight valence electrons for the main group elements, called an octet. When. Tin 2 Electrons Lost Ion.
From www.alamy.com
Sn Tin, Periodic Table of the Elements, Shell Structure of Tin Tin 2 Electrons Lost Ion So, these two electrons will be lost from the last 5p orbital. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e., usually eight valence electrons for the main group elements, called an octet. A cation (a positive ion) forms when a neutral atom loses one or. Tin 2 Electrons Lost Ion.
From valenceelectrons.com
How to find the Protons, Neutrons and Electrons for Tin? Tin 2 Electrons Lost Ion A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when. Tin and lead, though members of. Define the two types of ions. The tin electron configuration, represented as 5s 2 4d 10 5p 2 or 1s 2 2s 2 2p 6 3s 2. Tin 2 Electrons Lost Ion.
From valenceelectrons.com
Complete Electron Configuration for Tin (Sn, Sn2+, Sn4+) Tin 2 Electrons Lost Ion The tin electron configuration, represented as 5s 2 4d 10 5p 2 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2, illustrates the precise arrangement of electrons. During bond formation, the tin atom donates. Tin and lead, though members of. So, these two electrons will be. Tin 2 Electrons Lost Ion.
From periodictable.me
Tin Electron Configuration (Sn) with Orbital Diagram Tin 2 Electrons Lost Ion Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e., usually eight valence electrons for the main group elements, called an octet. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion). Tin 2 Electrons Lost Ion.
From www.slideserve.com
PPT 1 Name the ions formed by these elements and classify them as Tin 2 Electrons Lost Ion Define the two types of ions. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e., usually eight valence electrons for the main group elements, called an octet. It can sometimes lose two electrons to form the \(\ce{fe^{2+}}\) ion, while at other times it loses three electrons. Tin 2 Electrons Lost Ion.
From chem.libretexts.org
7.3 Lewis Symbols and Structures Chemistry LibreTexts Tin 2 Electrons Lost Ion The tin electron configuration, represented as 5s 2 4d 10 5p 2 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2, illustrates the precise arrangement of electrons. So, these two electrons will be lost from the last 5p orbital. During bond formation, the tin atom donates.. Tin 2 Electrons Lost Ion.
From www.slideserve.com
PPT Ions PowerPoint Presentation, free download ID6907887 Tin 2 Electrons Lost Ion Tin and lead, though members of. During bond formation, the tin atom donates. It can sometimes lose two electrons to form the \(\ce{fe^{2+}}\) ion, while at other times it loses three electrons to form the \(\ce{fe^{3+}}\) ion. The tin electron configuration, represented as 5s 2 4d 10 5p 2 or 1s 2 2s 2 2p 6 3s 2 3p 6. Tin 2 Electrons Lost Ion.
From periodictable.me
Tin Electron Configuration (Sn) with Orbital Diagram Tin 2 Electrons Lost Ion During bond formation, the tin atom donates. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e., usually eight valence electrons for the main group elements, called an octet. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to. Tin 2 Electrons Lost Ion.
From material-properties.org
Tin Periodic Table and Atomic Properties Tin 2 Electrons Lost Ion The tin electron configuration, represented as 5s 2 4d 10 5p 2 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2, illustrates the precise arrangement of electrons. Define the two types of ions. It can sometimes lose two electrons to form the \(\ce{fe^{2+}}\) ion, while at. Tin 2 Electrons Lost Ion.
From valenceelectrons.com
Protons, Neutrons, Electrons for Tin (Sn, Sn2+, Sn4+) Tin 2 Electrons Lost Ion Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. Tin and lead, though members of. When a tin atom loses two electrons it exhibits a +2 charge. It can sometimes lose two electrons to form the \(\ce{fe^{2+}}\) ion, while at other times it loses three electrons to form. Tin 2 Electrons Lost Ion.
From slideplayer.com
Ion Electron Method Ch ppt download Tin 2 Electrons Lost Ion The tin electron configuration, represented as 5s 2 4d 10 5p 2 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2, illustrates the precise arrangement of electrons. During bond formation, the tin atom donates. A cation (a positive ion) forms when a neutral atom loses one. Tin 2 Electrons Lost Ion.
From www.mooramo.com
Gaining and Losing Electrons Mooramo Tin 2 Electrons Lost Ion It can sometimes lose two electrons to form the \(\ce{fe^{2+}}\) ion, while at other times it loses three electrons to form the \(\ce{fe^{3+}}\) ion. When a tin atom loses two electrons it exhibits a +2 charge. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. A cation (a. Tin 2 Electrons Lost Ion.
From www.youtube.com
Sn^2+,Sn^4+ and Sn (Tin and Tin Ions) Electron ConfigurationCrash Tin 2 Electrons Lost Ion The tin electron configuration, represented as 5s 2 4d 10 5p 2 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2, illustrates the precise arrangement of electrons. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance. Tin 2 Electrons Lost Ion.
From www.slideserve.com
PPT UNIT 1 Structure and Function (Biochemistry) Chapter 2 Tin 2 Electrons Lost Ion So, these two electrons will be lost from the last 5p orbital. It can sometimes lose two electrons to form the \(\ce{fe^{2+}}\) ion, while at other times it loses three electrons to form the \(\ce{fe^{3+}}\) ion. Define the two types of ions. When a tin atom loses two electrons it exhibits a +2 charge. The tin electron configuration, represented as. Tin 2 Electrons Lost Ion.
From valenceelectrons.com
Electron Configuration for Tin and Tin ion(Sn2+, Sn4+) Tin 2 Electrons Lost Ion Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e., usually eight valence electrons for the main group elements, called an octet. Tin and lead, though members of. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance. Tin 2 Electrons Lost Ion.
From slideplayer.com
ELECTRON CONFIGURATION. ppt download Tin 2 Electrons Lost Ion Define the two types of ions. It can sometimes lose two electrons to form the \(\ce{fe^{2+}}\) ion, while at other times it loses three electrons to form the \(\ce{fe^{3+}}\) ion. The tin electron configuration, represented as 5s 2 4d 10 5p 2 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s. Tin 2 Electrons Lost Ion.
From www.youtube.com
How to Find the Valence Electrons for Tin (Sn) YouTube Tin 2 Electrons Lost Ion The tin electron configuration, represented as 5s 2 4d 10 5p 2 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2, illustrates the precise arrangement of electrons. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell,. Tin 2 Electrons Lost Ion.
From www.stonecoldhands.com
Bonds From Atoms Stone Cold Chemistry Talk Tin 2 Electrons Lost Ion During bond formation, the tin atom donates. The tin electron configuration, represented as 5s 2 4d 10 5p 2 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2, illustrates the precise arrangement of electrons. Atoms tend to lose, gain, or share some valance electrons, making bonds. Tin 2 Electrons Lost Ion.
From www.alamy.com
3d render of atom structure of tin isolated over white background Tin 2 Electrons Lost Ion A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when. So, these two electrons will be lost from the last 5p orbital. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive.. Tin 2 Electrons Lost Ion.