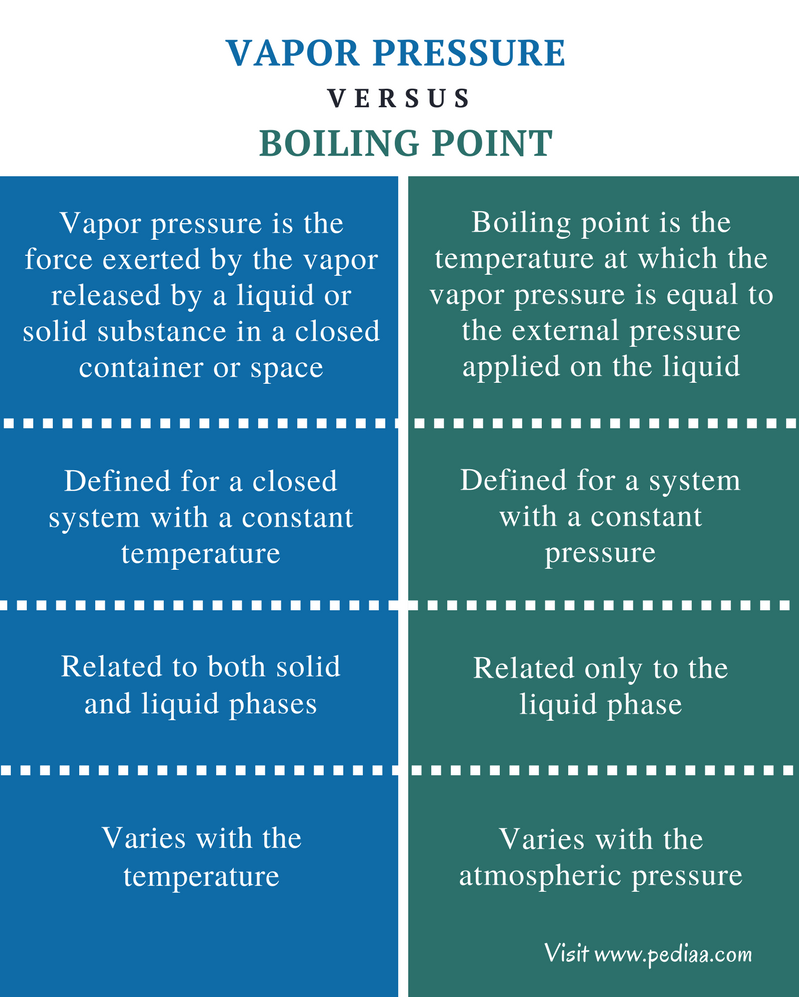
What Is A Boiling Point In Science . Boiling point is the temperature at which a liquid turns into vapour without changing its temperature. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. This is the boiling point. Boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. The normal boiling point of water is 100° c at sea level, but it. Boiling point is the temperature where a liquid's vapor pressure equals its applied pressure. Learn how altitude affects boiling. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. The change from a liquid phase to. The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid. Learn how boiling point depends on pressure, purity,.
from pediaa.com
If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. The normal boiling point of water is 100° c at sea level, but it. Learn how altitude affects boiling. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid. Boiling point is the temperature where a liquid's vapor pressure equals its applied pressure. Learn how boiling point depends on pressure, purity,. The change from a liquid phase to. This is the boiling point. Boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure.
Difference Between Vapor Pressure and Boiling Point Definition
What Is A Boiling Point In Science The normal boiling point of water is 100° c at sea level, but it. The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid. The change from a liquid phase to. Boiling point is the temperature at which a liquid turns into vapour without changing its temperature. The normal boiling point of water is 100° c at sea level, but it. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. This is the boiling point. Boiling point is the temperature where a liquid's vapor pressure equals its applied pressure. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. Learn how boiling point depends on pressure, purity,. Learn how altitude affects boiling. Boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure.
From www.pinterest.co.uk
Boiling Point Elevation Easy Science Easy science, Boiling point What Is A Boiling Point In Science Learn how altitude affects boiling. The normal boiling point of water is 100° c at sea level, but it. Boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. Boiling point is the temperature at which a liquid turns into vapour without changing its temperature. The boiling point is the temperature. What Is A Boiling Point In Science.
From www.pinterest.com
Boiling Point/Freezing/ Melting Point AnchorChart Teaching science What Is A Boiling Point In Science Boiling point is the temperature where a liquid's vapor pressure equals its applied pressure. Learn how altitude affects boiling. This is the boiling point. Boiling point is the temperature at which a liquid turns into vapour without changing its temperature. The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the. What Is A Boiling Point In Science.
From www.thoughtco.com
Normal Boiling Point Definition (Chemistry) What Is A Boiling Point In Science Learn how boiling point depends on pressure, purity,. This is the boiling point. Learn how altitude affects boiling. Boiling point is the temperature at which a liquid turns into vapour without changing its temperature. Boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. The normal boiling point of water is. What Is A Boiling Point In Science.
From byjus.com
What is the difference between melting point and boiling point What Is A Boiling Point In Science Learn how altitude affects boiling. Boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. The change from a liquid phase to. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. The normal boiling point of water is 100° c. What Is A Boiling Point In Science.
From sciencenotes.org
Boiling Point of Water What Temperature Does Water Boil? What Is A Boiling Point In Science Learn how boiling point depends on pressure, purity,. The normal boiling point of water is 100° c at sea level, but it. This is the boiling point. The change from a liquid phase to. The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid. Boiling point is the temperature. What Is A Boiling Point In Science.
From www.youtube.com
Melting and Boiling Points p98 (Foundation p97) YouTube What Is A Boiling Point In Science If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. The normal boiling point of water is 100° c at sea level, but it. Boiling is the process by which a liquid turns into a vapor when it is heated to its. What Is A Boiling Point In Science.
From www.teachoo.com
What is the Difference between Evaporation and Boiling? Class 9 What Is A Boiling Point In Science The normal boiling point of water is 100° c at sea level, but it. This is the boiling point. Learn how boiling point depends on pressure, purity,. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. The boiling point is the temperature at which the vapor pressure of a. What Is A Boiling Point In Science.
From favpng.com
Boiling Point Melting Point Heat Temperature Chemistry, PNG What Is A Boiling Point In Science The normal boiling point of water is 100° c at sea level, but it. The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its. What Is A Boiling Point In Science.
From www.healthbenefitstimes.com
Boiling Point Definition of Boiling Point What Is A Boiling Point In Science Boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. Boiling point is the temperature at which a liquid turns into vapour without changing its temperature. The change from a liquid phase to. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the. What Is A Boiling Point In Science.
From facts.net
20 Fascinating Facts About Boiling Point Elevation What Is A Boiling Point In Science Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. The normal boiling point of water is 100° c at sea level, but it. Boiling point is the temperature at which a liquid turns into vapour without changing its temperature. Boiling point is the temperature where a liquid's vapor pressure. What Is A Boiling Point In Science.
From www.slideserve.com
PPT States of Matter Phase Change PowerPoint Presentation ID1115834 What Is A Boiling Point In Science Boiling point is the temperature at which a liquid turns into vapour without changing its temperature. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. The normal boiling point of water is 100° c at sea level, but it. Learn how altitude affects boiling. Learn how boiling point depends. What Is A Boiling Point In Science.
From www.vedantu.com
Boiling Point Elevation Learn Important Terms and Concepts What Is A Boiling Point In Science Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. Boiling point is the temperature where a liquid's vapor pressure equals its applied pressure. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal. What Is A Boiling Point In Science.
From sciencenotes.org
Boiling Point Definition, Temperature, and Examples What Is A Boiling Point In Science Boiling point is the temperature at which a liquid turns into vapour without changing its temperature. The normal boiling point of water is 100° c at sea level, but it. Boiling point is the temperature where a liquid's vapor pressure equals its applied pressure. The boiling point is the temperature at which the vapor pressure of a liquid equals the. What Is A Boiling Point In Science.
From www.dreamstime.com
Boiling and Evaporation, Freezing and Melting Points of Water Stock What Is A Boiling Point In Science Boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. The change from a liquid phase to. Learn how altitude affects boiling. Boiling point is the temperature where a liquid's vapor pressure equals its applied pressure. Boiling is the process by which a liquid turns into a vapor when it is. What Is A Boiling Point In Science.
From aweseas.blogspot.com
Boiling Point Of Water At Sea Level In Kelvin What Is A Boiling Point In Science Boiling point is the temperature at which a liquid turns into vapour without changing its temperature. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. Learn how boiling point depends on pressure, purity,. This is the boiling point. The normal boiling. What Is A Boiling Point In Science.
From ppt-online.org
theory of ideal gases презентация онлайн What Is A Boiling Point In Science The normal boiling point of water is 100° c at sea level, but it. The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. If this pressure is the. What Is A Boiling Point In Science.
From www.slideserve.com
PPT Chapter 12 PowerPoint Presentation, free download ID1624745 What Is A Boiling Point In Science Learn how boiling point depends on pressure, purity,. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. The change from a liquid phase to. The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid. Boiling point is the. What Is A Boiling Point In Science.
From gamesmartz.com
Boiling Point Definition & Image GameSmartz What Is A Boiling Point In Science This is the boiling point. The normal boiling point of water is 100° c at sea level, but it. The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid. The change from a liquid phase to. Learn how altitude affects boiling. Boiling point is the temperature at which a. What Is A Boiling Point In Science.
From www.youtube.com
What is Boiling Point Concept of Boiling Point Boiling Point What Is A Boiling Point In Science Boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. This is the boiling point. Learn how altitude affects boiling. Boiling point is the temperature at which a liquid turns into vapour without changing its temperature. Boiling is the process by which a liquid turns into a vapor when it is. What Is A Boiling Point In Science.
From marshallkruwmathis.blogspot.com
What is the Boiling Point of Water MarshallkruwMathis What Is A Boiling Point In Science Boiling point is the temperature at which a liquid turns into vapour without changing its temperature. This is the boiling point. Boiling point is the temperature where a liquid's vapor pressure equals its applied pressure. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its. What Is A Boiling Point In Science.
From sciencenotes.org
Boiling Point Definition, Temperature, and Examples What Is A Boiling Point In Science The normal boiling point of water is 100° c at sea level, but it. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. Boiling point is the temperature at which a liquid turns into vapour without changing its temperature. Boiling point is the temperature where a liquid's vapor pressure. What Is A Boiling Point In Science.
From www.slideserve.com
PPT boiling point PowerPoint Presentation, free download ID2402961 What Is A Boiling Point In Science Boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. The normal boiling point of water is 100° c at sea level, but it. This is the boiling point. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred. What Is A Boiling Point In Science.
From sciencenotes.org
What Are the Bubbles in Boiling Water? What Is A Boiling Point In Science Learn how altitude affects boiling. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. This is the boiling point. Boiling point is the temperature where a liquid's vapor pressure equals its applied pressure. Boiling point is the temperature at which the. What Is A Boiling Point In Science.
From www.slideserve.com
PPT Boiling Point Notes PowerPoint Presentation, free download ID What Is A Boiling Point In Science Learn how altitude affects boiling. This is the boiling point. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. The normal boiling point of water is 100° c at sea level, but it. Boiling point is the temperature at which the. What Is A Boiling Point In Science.
From www.gettyimages.ie
Freezing And Boiling Points In Celsius And Fahrenheit HighRes Vector What Is A Boiling Point In Science The change from a liquid phase to. Boiling point is the temperature at which a liquid turns into vapour without changing its temperature. This is the boiling point. Boiling point is the temperature where a liquid's vapor pressure equals its applied pressure. Learn how boiling point depends on pressure, purity,. The boiling point is the temperature at which the vapor. What Is A Boiling Point In Science.
From www.slideserve.com
PPT Properties and Changes in Matter PowerPoint Presentation, free What Is A Boiling Point In Science Learn how boiling point depends on pressure, purity,. This is the boiling point. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling. What Is A Boiling Point In Science.
From gamesmartz.com
Normal Boiling Point Definition & Image GameSmartz What Is A Boiling Point In Science Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid. What Is A Boiling Point In Science.
From www.slideserve.com
PPT Freezing/Melting and Boiling Points PowerPoint Presentation, free What Is A Boiling Point In Science This is the boiling point. Boiling point is the temperature where a liquid's vapor pressure equals its applied pressure. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. Learn how altitude affects boiling. The normal boiling point of water is 100°. What Is A Boiling Point In Science.
From stock.adobe.com
Boiling and Evaporation, Freezing and Melting Points of Water. Stock What Is A Boiling Point In Science Boiling point is the temperature where a liquid's vapor pressure equals its applied pressure. The change from a liquid phase to. Learn how altitude affects boiling. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the. What Is A Boiling Point In Science.
From www.worksheetsplanet.com
What is Boiling Point What Is A Boiling Point In Science Boiling point is the temperature at which a liquid turns into vapour without changing its temperature. The change from a liquid phase to. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. This is the boiling point. Boiling point is the. What Is A Boiling Point In Science.
From pediaa.com
Difference Between Vapor Pressure and Boiling Point Definition What Is A Boiling Point In Science If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. Boiling point is the temperature where a liquid's vapor pressure equals its applied pressure. Learn how boiling point depends on pressure, purity,. The normal boiling point of water is 100° c at. What Is A Boiling Point In Science.
From www.animalia-life.club
Boiling Point Of Water For Kids What Is A Boiling Point In Science Boiling point is the temperature where a liquid's vapor pressure equals its applied pressure. Boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. The change from a liquid phase to. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point.. What Is A Boiling Point In Science.
From www.slideserve.com
PPT Melting Point and Boiling Point PowerPoint Presentation, free What Is A Boiling Point In Science Boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. Learn how boiling point depends on pressure, purity,. Learn how altitude affects boiling. The normal boiling point of water is 100° c at sea level, but it. Boiling is the process by which a liquid turns into a vapor when it. What Is A Boiling Point In Science.
From www.slideserve.com
PPT PROPERTIES PowerPoint Presentation, free download ID6387884 What Is A Boiling Point In Science The change from a liquid phase to. Boiling point is the temperature at which a liquid turns into vapour without changing its temperature. Boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. Learn how boiling point depends on pressure, purity,. Learn how altitude affects boiling. If this pressure is the. What Is A Boiling Point In Science.
From www.learnatnoon.com
The boiling point of water and alcohol explained Noon Academy What Is A Boiling Point In Science Learn how boiling point depends on pressure, purity,. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. Boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. The normal boiling point of. What Is A Boiling Point In Science.