Protein Ultraviolet Absorption Spectroscopy . The most frequently employed spectral range for proteins is between 250 and 320 nm, a region referred to as the near. A ratio of absorbances at 250, 275, and 280 nm is sensitive to protein structure. Quantitation of the amount of protein in a solution is possible in a simple spectrometer. Apart from their intrinsic absorptivity, proteins will absorb uv light in proportion to their concentrations. 3 uv spectra of proteins. The optical activity of proteins in the near uv is directly related to the electronic structure and optical absorption of aromatic. Protein concentration measurement by uv concentration of a purified protein is best measured spectrophotometrically using. This relationship has been exploited for the spectrophotometric. Absorption of radiation in the near uv by proteins depends. The ratio is useful as probe of. Proteins primarily absorb uv light due to the presence of tryptophan, tyrosine, and phenylalanine residues, with.
from www.semanticscholar.org
3 uv spectra of proteins. Proteins primarily absorb uv light due to the presence of tryptophan, tyrosine, and phenylalanine residues, with. This relationship has been exploited for the spectrophotometric. The most frequently employed spectral range for proteins is between 250 and 320 nm, a region referred to as the near. Apart from their intrinsic absorptivity, proteins will absorb uv light in proportion to their concentrations. Quantitation of the amount of protein in a solution is possible in a simple spectrometer. A ratio of absorbances at 250, 275, and 280 nm is sensitive to protein structure. The optical activity of proteins in the near uv is directly related to the electronic structure and optical absorption of aromatic. The ratio is useful as probe of. Absorption of radiation in the near uv by proteins depends.
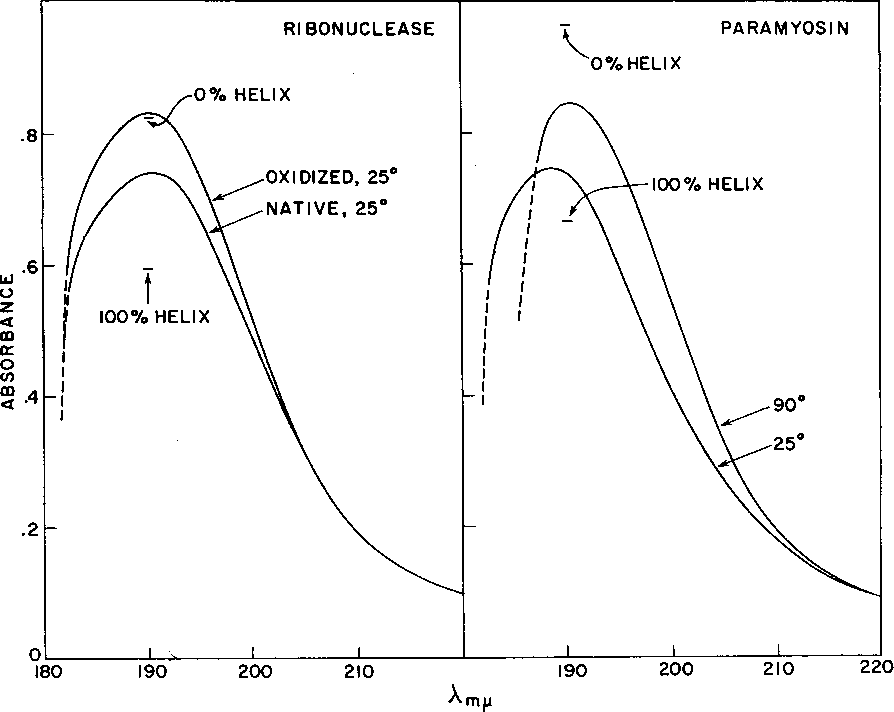
Figure 6 from The far ultraviolet absorption spectra of polypeptide and
Protein Ultraviolet Absorption Spectroscopy Absorption of radiation in the near uv by proteins depends. The most frequently employed spectral range for proteins is between 250 and 320 nm, a region referred to as the near. Absorption of radiation in the near uv by proteins depends. This relationship has been exploited for the spectrophotometric. Protein concentration measurement by uv concentration of a purified protein is best measured spectrophotometrically using. Apart from their intrinsic absorptivity, proteins will absorb uv light in proportion to their concentrations. Proteins primarily absorb uv light due to the presence of tryptophan, tyrosine, and phenylalanine residues, with. The optical activity of proteins in the near uv is directly related to the electronic structure and optical absorption of aromatic. Quantitation of the amount of protein in a solution is possible in a simple spectrometer. The ratio is useful as probe of. A ratio of absorbances at 250, 275, and 280 nm is sensitive to protein structure. 3 uv spectra of proteins.
From www.researchgate.net
Ultraviolet and visible absorption spectroscopy (UVVis) of all Protein Ultraviolet Absorption Spectroscopy The optical activity of proteins in the near uv is directly related to the electronic structure and optical absorption of aromatic. 3 uv spectra of proteins. A ratio of absorbances at 250, 275, and 280 nm is sensitive to protein structure. Protein concentration measurement by uv concentration of a purified protein is best measured spectrophotometrically using. The ratio is useful. Protein Ultraviolet Absorption Spectroscopy.
From www.researchgate.net
A, ultravioletvisible absorption spectrum of the purified Protein Ultraviolet Absorption Spectroscopy Absorption of radiation in the near uv by proteins depends. The most frequently employed spectral range for proteins is between 250 and 320 nm, a region referred to as the near. The ratio is useful as probe of. This relationship has been exploited for the spectrophotometric. 3 uv spectra of proteins. The optical activity of proteins in the near uv. Protein Ultraviolet Absorption Spectroscopy.
From chem.libretexts.org
4.5 Ultraviolet and visible spectroscopy Chemistry LibreTexts Protein Ultraviolet Absorption Spectroscopy Quantitation of the amount of protein in a solution is possible in a simple spectrometer. 3 uv spectra of proteins. Apart from their intrinsic absorptivity, proteins will absorb uv light in proportion to their concentrations. Absorption of radiation in the near uv by proteins depends. The most frequently employed spectral range for proteins is between 250 and 320 nm, a. Protein Ultraviolet Absorption Spectroscopy.
From www.researchgate.net
Ultraviolet absorption spectrum of PNP at various pH values. The Protein Ultraviolet Absorption Spectroscopy Absorption of radiation in the near uv by proteins depends. A ratio of absorbances at 250, 275, and 280 nm is sensitive to protein structure. Quantitation of the amount of protein in a solution is possible in a simple spectrometer. Protein concentration measurement by uv concentration of a purified protein is best measured spectrophotometrically using. Apart from their intrinsic absorptivity,. Protein Ultraviolet Absorption Spectroscopy.
From www.researchgate.net
A Absorption spectra of DNA and proteins, with emission spectra of a Protein Ultraviolet Absorption Spectroscopy 3 uv spectra of proteins. This relationship has been exploited for the spectrophotometric. Quantitation of the amount of protein in a solution is possible in a simple spectrometer. Protein concentration measurement by uv concentration of a purified protein is best measured spectrophotometrically using. The most frequently employed spectral range for proteins is between 250 and 320 nm, a region referred. Protein Ultraviolet Absorption Spectroscopy.
From chem.libretexts.org
Ultraviolet and visible spectroscopy Chemistry LibreTexts Protein Ultraviolet Absorption Spectroscopy Proteins primarily absorb uv light due to the presence of tryptophan, tyrosine, and phenylalanine residues, with. The optical activity of proteins in the near uv is directly related to the electronic structure and optical absorption of aromatic. 3 uv spectra of proteins. Apart from their intrinsic absorptivity, proteins will absorb uv light in proportion to their concentrations. Absorption of radiation. Protein Ultraviolet Absorption Spectroscopy.
From www.youtube.com
What is Effect of Solvent on UV Absorption Spectra Spectroscopy Protein Ultraviolet Absorption Spectroscopy Proteins primarily absorb uv light due to the presence of tryptophan, tyrosine, and phenylalanine residues, with. This relationship has been exploited for the spectrophotometric. Apart from their intrinsic absorptivity, proteins will absorb uv light in proportion to their concentrations. Quantitation of the amount of protein in a solution is possible in a simple spectrometer. The ratio is useful as probe. Protein Ultraviolet Absorption Spectroscopy.
From www.researchgate.net
The ultraviolet absorption spectroscopy results, structures and Protein Ultraviolet Absorption Spectroscopy Protein concentration measurement by uv concentration of a purified protein is best measured spectrophotometrically using. Absorption of radiation in the near uv by proteins depends. Apart from their intrinsic absorptivity, proteins will absorb uv light in proportion to their concentrations. Proteins primarily absorb uv light due to the presence of tryptophan, tyrosine, and phenylalanine residues, with. This relationship has been. Protein Ultraviolet Absorption Spectroscopy.
From www.chem.ucla.edu
Illustrated Glossary of Organic Chemistry Ultraviolet spectroscopy Protein Ultraviolet Absorption Spectroscopy The optical activity of proteins in the near uv is directly related to the electronic structure and optical absorption of aromatic. Absorption of radiation in the near uv by proteins depends. Quantitation of the amount of protein in a solution is possible in a simple spectrometer. The most frequently employed spectral range for proteins is between 250 and 320 nm,. Protein Ultraviolet Absorption Spectroscopy.
From www.researchgate.net
(a) Ultravioletvisible absorption spectroscopy of (C 8 H 17 NH 2 ) 2 Protein Ultraviolet Absorption Spectroscopy Protein concentration measurement by uv concentration of a purified protein is best measured spectrophotometrically using. The ratio is useful as probe of. 3 uv spectra of proteins. A ratio of absorbances at 250, 275, and 280 nm is sensitive to protein structure. This relationship has been exploited for the spectrophotometric. Proteins primarily absorb uv light due to the presence of. Protein Ultraviolet Absorption Spectroscopy.
From www.researchgate.net
(A). Ultraviolet absorption spectrum of DNA in the presence of 10 mM Protein Ultraviolet Absorption Spectroscopy Protein concentration measurement by uv concentration of a purified protein is best measured spectrophotometrically using. The optical activity of proteins in the near uv is directly related to the electronic structure and optical absorption of aromatic. The ratio is useful as probe of. The most frequently employed spectral range for proteins is between 250 and 320 nm, a region referred. Protein Ultraviolet Absorption Spectroscopy.
From www.researchgate.net
Ultravioletvisible absorption spectroscopy of DOM components when the Protein Ultraviolet Absorption Spectroscopy Apart from their intrinsic absorptivity, proteins will absorb uv light in proportion to their concentrations. Absorption of radiation in the near uv by proteins depends. Proteins primarily absorb uv light due to the presence of tryptophan, tyrosine, and phenylalanine residues, with. Protein concentration measurement by uv concentration of a purified protein is best measured spectrophotometrically using. The ratio is useful. Protein Ultraviolet Absorption Spectroscopy.
From www.researchgate.net
The ultraviolet absorption spectrum of DBP solution with reaction time Protein Ultraviolet Absorption Spectroscopy Apart from their intrinsic absorptivity, proteins will absorb uv light in proportion to their concentrations. A ratio of absorbances at 250, 275, and 280 nm is sensitive to protein structure. The most frequently employed spectral range for proteins is between 250 and 320 nm, a region referred to as the near. This relationship has been exploited for the spectrophotometric. Protein. Protein Ultraviolet Absorption Spectroscopy.
From www.researchgate.net
The ultravioletvisible absorption spectra of antigen. (a) Immunogen Protein Ultraviolet Absorption Spectroscopy 3 uv spectra of proteins. The ratio is useful as probe of. The most frequently employed spectral range for proteins is between 250 and 320 nm, a region referred to as the near. Proteins primarily absorb uv light due to the presence of tryptophan, tyrosine, and phenylalanine residues, with. Protein concentration measurement by uv concentration of a purified protein is. Protein Ultraviolet Absorption Spectroscopy.
From chem.libretexts.org
4.3 Ultraviolet and visible spectroscopy Chemistry LibreTexts Protein Ultraviolet Absorption Spectroscopy Apart from their intrinsic absorptivity, proteins will absorb uv light in proportion to their concentrations. Protein concentration measurement by uv concentration of a purified protein is best measured spectrophotometrically using. Quantitation of the amount of protein in a solution is possible in a simple spectrometer. Proteins primarily absorb uv light due to the presence of tryptophan, tyrosine, and phenylalanine residues,. Protein Ultraviolet Absorption Spectroscopy.
From www.researchgate.net
Ultraviolet absorption spectra of PDPA with different contents of NiO Protein Ultraviolet Absorption Spectroscopy Apart from their intrinsic absorptivity, proteins will absorb uv light in proportion to their concentrations. Quantitation of the amount of protein in a solution is possible in a simple spectrometer. This relationship has been exploited for the spectrophotometric. Absorption of radiation in the near uv by proteins depends. 3 uv spectra of proteins. The optical activity of proteins in the. Protein Ultraviolet Absorption Spectroscopy.
From www.researchgate.net
Representative UV absorption spectra from 195 to 300 nm. (a) DMPC Protein Ultraviolet Absorption Spectroscopy The ratio is useful as probe of. A ratio of absorbances at 250, 275, and 280 nm is sensitive to protein structure. The optical activity of proteins in the near uv is directly related to the electronic structure and optical absorption of aromatic. The most frequently employed spectral range for proteins is between 250 and 320 nm, a region referred. Protein Ultraviolet Absorption Spectroscopy.
From www.researchgate.net
Absorption spectrum of the pigmentprotein complex. Download Protein Ultraviolet Absorption Spectroscopy Absorption of radiation in the near uv by proteins depends. The most frequently employed spectral range for proteins is between 250 and 320 nm, a region referred to as the near. Quantitation of the amount of protein in a solution is possible in a simple spectrometer. The optical activity of proteins in the near uv is directly related to the. Protein Ultraviolet Absorption Spectroscopy.
From www.researchgate.net
Ultravioletvisible spectroscopy (UVVis) absorption and emission Protein Ultraviolet Absorption Spectroscopy Proteins primarily absorb uv light due to the presence of tryptophan, tyrosine, and phenylalanine residues, with. The ratio is useful as probe of. Absorption of radiation in the near uv by proteins depends. A ratio of absorbances at 250, 275, and 280 nm is sensitive to protein structure. Quantitation of the amount of protein in a solution is possible in. Protein Ultraviolet Absorption Spectroscopy.
From www.researchgate.net
The UVvis absorption spectra of proteinAuNP conjugations in the Protein Ultraviolet Absorption Spectroscopy The optical activity of proteins in the near uv is directly related to the electronic structure and optical absorption of aromatic. This relationship has been exploited for the spectrophotometric. 3 uv spectra of proteins. Proteins primarily absorb uv light due to the presence of tryptophan, tyrosine, and phenylalanine residues, with. The ratio is useful as probe of. The most frequently. Protein Ultraviolet Absorption Spectroscopy.
From www.researchgate.net
Representative UV absorption spectra from 195 to 300 nm. (a) DMPC Protein Ultraviolet Absorption Spectroscopy Quantitation of the amount of protein in a solution is possible in a simple spectrometer. 3 uv spectra of proteins. The optical activity of proteins in the near uv is directly related to the electronic structure and optical absorption of aromatic. The ratio is useful as probe of. Proteins primarily absorb uv light due to the presence of tryptophan, tyrosine,. Protein Ultraviolet Absorption Spectroscopy.
From www.youtube.com
Proteins UV Vis Spectroscopy Analysis YouTube Protein Ultraviolet Absorption Spectroscopy Absorption of radiation in the near uv by proteins depends. Quantitation of the amount of protein in a solution is possible in a simple spectrometer. The optical activity of proteins in the near uv is directly related to the electronic structure and optical absorption of aromatic. The most frequently employed spectral range for proteins is between 250 and 320 nm,. Protein Ultraviolet Absorption Spectroscopy.
From www.researchgate.net
Normalized ultravioletvisible (UVvis) absorption spectra of P3HT (a Protein Ultraviolet Absorption Spectroscopy The ratio is useful as probe of. Protein concentration measurement by uv concentration of a purified protein is best measured spectrophotometrically using. Apart from their intrinsic absorptivity, proteins will absorb uv light in proportion to their concentrations. This relationship has been exploited for the spectrophotometric. The most frequently employed spectral range for proteins is between 250 and 320 nm, a. Protein Ultraviolet Absorption Spectroscopy.
From www.researchgate.net
UVVis absorption spectrum of copper(III)periodate complex. Blue Protein Ultraviolet Absorption Spectroscopy Protein concentration measurement by uv concentration of a purified protein is best measured spectrophotometrically using. Proteins primarily absorb uv light due to the presence of tryptophan, tyrosine, and phenylalanine residues, with. Absorption of radiation in the near uv by proteins depends. This relationship has been exploited for the spectrophotometric. The optical activity of proteins in the near uv is directly. Protein Ultraviolet Absorption Spectroscopy.
From chem.libretexts.org
4.5 Ultraviolet and visible spectroscopy Chemistry LibreTexts Protein Ultraviolet Absorption Spectroscopy The optical activity of proteins in the near uv is directly related to the electronic structure and optical absorption of aromatic. The most frequently employed spectral range for proteins is between 250 and 320 nm, a region referred to as the near. Protein concentration measurement by uv concentration of a purified protein is best measured spectrophotometrically using. Proteins primarily absorb. Protein Ultraviolet Absorption Spectroscopy.
From www3.nd.edu
UV absorbance spectra of the three aromatic amino acids, phenylalanine Protein Ultraviolet Absorption Spectroscopy Quantitation of the amount of protein in a solution is possible in a simple spectrometer. The optical activity of proteins in the near uv is directly related to the electronic structure and optical absorption of aromatic. Protein concentration measurement by uv concentration of a purified protein is best measured spectrophotometrically using. This relationship has been exploited for the spectrophotometric. The. Protein Ultraviolet Absorption Spectroscopy.
From www.researchgate.net
Absorption spectra of various proteins. Download Scientific Diagram Protein Ultraviolet Absorption Spectroscopy Protein concentration measurement by uv concentration of a purified protein is best measured spectrophotometrically using. Proteins primarily absorb uv light due to the presence of tryptophan, tyrosine, and phenylalanine residues, with. A ratio of absorbances at 250, 275, and 280 nm is sensitive to protein structure. Absorption of radiation in the near uv by proteins depends. The optical activity of. Protein Ultraviolet Absorption Spectroscopy.
From www.microspectra.com
Ultraviolet Absorption Spectroscopy Proteins Supplier Protein Ultraviolet Absorption Spectroscopy The optical activity of proteins in the near uv is directly related to the electronic structure and optical absorption of aromatic. 3 uv spectra of proteins. The ratio is useful as probe of. Quantitation of the amount of protein in a solution is possible in a simple spectrometer. This relationship has been exploited for the spectrophotometric. Absorption of radiation in. Protein Ultraviolet Absorption Spectroscopy.
From www.researchgate.net
Ultraviolet absorption spectra of the assynthesized nanobelts and Protein Ultraviolet Absorption Spectroscopy Apart from their intrinsic absorptivity, proteins will absorb uv light in proportion to their concentrations. Protein concentration measurement by uv concentration of a purified protein is best measured spectrophotometrically using. Proteins primarily absorb uv light due to the presence of tryptophan, tyrosine, and phenylalanine residues, with. 3 uv spectra of proteins. Absorption of radiation in the near uv by proteins. Protein Ultraviolet Absorption Spectroscopy.
From www.kemtrak.com
Protein measurement Application note Kemtrak Protein Ultraviolet Absorption Spectroscopy The ratio is useful as probe of. Protein concentration measurement by uv concentration of a purified protein is best measured spectrophotometrically using. 3 uv spectra of proteins. Apart from their intrinsic absorptivity, proteins will absorb uv light in proportion to their concentrations. Quantitation of the amount of protein in a solution is possible in a simple spectrometer. The most frequently. Protein Ultraviolet Absorption Spectroscopy.
From www.researchgate.net
Ultraviolet absorption spectra of bran protein hydrolysates. VPI and Protein Ultraviolet Absorption Spectroscopy The most frequently employed spectral range for proteins is between 250 and 320 nm, a region referred to as the near. Proteins primarily absorb uv light due to the presence of tryptophan, tyrosine, and phenylalanine residues, with. 3 uv spectra of proteins. This relationship has been exploited for the spectrophotometric. A ratio of absorbances at 250, 275, and 280 nm. Protein Ultraviolet Absorption Spectroscopy.
From chem.libretexts.org
4.5 Ultraviolet and visible spectroscopy Chemistry LibreTexts Protein Ultraviolet Absorption Spectroscopy The optical activity of proteins in the near uv is directly related to the electronic structure and optical absorption of aromatic. 3 uv spectra of proteins. The ratio is useful as probe of. The most frequently employed spectral range for proteins is between 250 and 320 nm, a region referred to as the near. Proteins primarily absorb uv light due. Protein Ultraviolet Absorption Spectroscopy.
From www.slideserve.com
PPT Molecular UVVisible Spectroscopy PowerPoint Presentation, free Protein Ultraviolet Absorption Spectroscopy Apart from their intrinsic absorptivity, proteins will absorb uv light in proportion to their concentrations. A ratio of absorbances at 250, 275, and 280 nm is sensitive to protein structure. The ratio is useful as probe of. Protein concentration measurement by uv concentration of a purified protein is best measured spectrophotometrically using. The optical activity of proteins in the near. Protein Ultraviolet Absorption Spectroscopy.
From www.researchgate.net
Ultravioletvisible spectroscopy (UV−Vis) absorption and... Download Protein Ultraviolet Absorption Spectroscopy The ratio is useful as probe of. Absorption of radiation in the near uv by proteins depends. Protein concentration measurement by uv concentration of a purified protein is best measured spectrophotometrically using. This relationship has been exploited for the spectrophotometric. Proteins primarily absorb uv light due to the presence of tryptophan, tyrosine, and phenylalanine residues, with. The optical activity of. Protein Ultraviolet Absorption Spectroscopy.
From www.semanticscholar.org
Figure 6 from The far ultraviolet absorption spectra of polypeptide and Protein Ultraviolet Absorption Spectroscopy The optical activity of proteins in the near uv is directly related to the electronic structure and optical absorption of aromatic. This relationship has been exploited for the spectrophotometric. Apart from their intrinsic absorptivity, proteins will absorb uv light in proportion to their concentrations. Quantitation of the amount of protein in a solution is possible in a simple spectrometer. The. Protein Ultraviolet Absorption Spectroscopy.