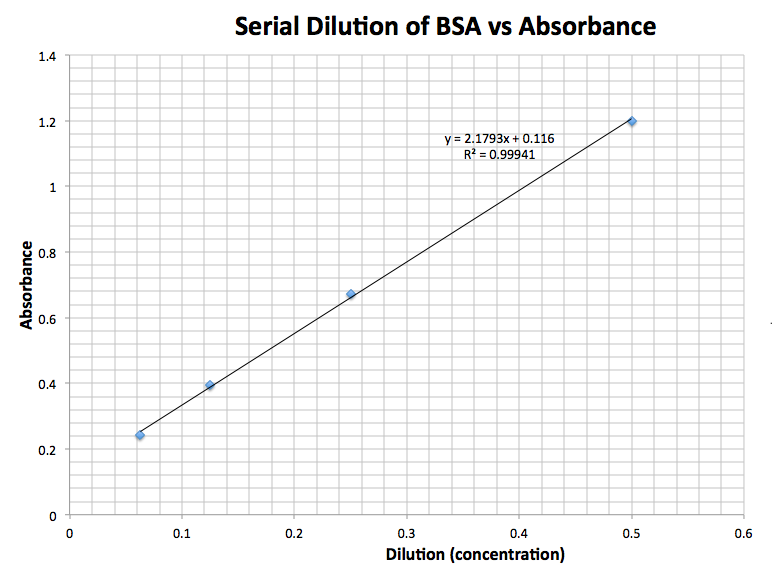
What Is The Purpose Of A Standard Curve In Spectrophotometry . A standard curve translates absorbance values into concentration. Spectrophotometers are instruments designed to detect the amount of light energy that is absorbed or transmitted by. Standard curves are graphs of light absorbance versus solution concentration which can be used to figure out the solute concentration in unknown samples. Spectrophotometry is a method to measure how much a chemical substance absorbs light by measuring the intensity of light as a beam of light passes through sample solution. Curve of light absorbance and transmission. See fig 2.1 for an example. Standard curves (also known as calibration curves) represent the relationship between two quantities. Every standard curve is generated using a. The standard curve will be used in.
from ambitiousmares.blogspot.com
See fig 2.1 for an example. Standard curves (also known as calibration curves) represent the relationship between two quantities. The standard curve will be used in. Spectrophotometers are instruments designed to detect the amount of light energy that is absorbed or transmitted by. A standard curve translates absorbance values into concentration. Curve of light absorbance and transmission. Spectrophotometry is a method to measure how much a chemical substance absorbs light by measuring the intensity of light as a beam of light passes through sample solution. Standard curves are graphs of light absorbance versus solution concentration which can be used to figure out the solute concentration in unknown samples. Every standard curve is generated using a.
32 Label The Graph Below With The Correct Axes For A Standard Curve
What Is The Purpose Of A Standard Curve In Spectrophotometry The standard curve will be used in. See fig 2.1 for an example. The standard curve will be used in. Spectrophotometry is a method to measure how much a chemical substance absorbs light by measuring the intensity of light as a beam of light passes through sample solution. Every standard curve is generated using a. Curve of light absorbance and transmission. Standard curves are graphs of light absorbance versus solution concentration which can be used to figure out the solute concentration in unknown samples. Spectrophotometers are instruments designed to detect the amount of light energy that is absorbed or transmitted by. Standard curves (also known as calibration curves) represent the relationship between two quantities. A standard curve translates absorbance values into concentration.
From mungfali.com
Standard Curve Graph What Is The Purpose Of A Standard Curve In Spectrophotometry Standard curves (also known as calibration curves) represent the relationship between two quantities. A standard curve translates absorbance values into concentration. See fig 2.1 for an example. Every standard curve is generated using a. The standard curve will be used in. Spectrophotometers are instruments designed to detect the amount of light energy that is absorbed or transmitted by. Curve of. What Is The Purpose Of A Standard Curve In Spectrophotometry.
From www.researchgate.net
Example of a qPCR standard curve. Download Scientific Diagram What Is The Purpose Of A Standard Curve In Spectrophotometry A standard curve translates absorbance values into concentration. Standard curves are graphs of light absorbance versus solution concentration which can be used to figure out the solute concentration in unknown samples. The standard curve will be used in. Every standard curve is generated using a. Spectrophotometers are instruments designed to detect the amount of light energy that is absorbed or. What Is The Purpose Of A Standard Curve In Spectrophotometry.
From www.slideserve.com
PPT Spectrophotometry PowerPoint Presentation, free download ID6911139 What Is The Purpose Of A Standard Curve In Spectrophotometry Spectrophotometry is a method to measure how much a chemical substance absorbs light by measuring the intensity of light as a beam of light passes through sample solution. Spectrophotometers are instruments designed to detect the amount of light energy that is absorbed or transmitted by. The standard curve will be used in. Standard curves are graphs of light absorbance versus. What Is The Purpose Of A Standard Curve In Spectrophotometry.
From ceofpqeq.blob.core.windows.net
Absorption Spectrophotometer Uses at Luciano blog What Is The Purpose Of A Standard Curve In Spectrophotometry The standard curve will be used in. Every standard curve is generated using a. Standard curves are graphs of light absorbance versus solution concentration which can be used to figure out the solute concentration in unknown samples. See fig 2.1 for an example. Curve of light absorbance and transmission. Standard curves (also known as calibration curves) represent the relationship between. What Is The Purpose Of A Standard Curve In Spectrophotometry.
From www.researchgate.net
Representative of standard curve illustrating the peak area ratio (PAR What Is The Purpose Of A Standard Curve In Spectrophotometry Standard curves are graphs of light absorbance versus solution concentration which can be used to figure out the solute concentration in unknown samples. Every standard curve is generated using a. Spectrophotometers are instruments designed to detect the amount of light energy that is absorbed or transmitted by. Standard curves (also known as calibration curves) represent the relationship between two quantities.. What Is The Purpose Of A Standard Curve In Spectrophotometry.
From www.scielo.br
SciELO Brasil Use of different matrices to construct the standard What Is The Purpose Of A Standard Curve In Spectrophotometry Curve of light absorbance and transmission. See fig 2.1 for an example. Every standard curve is generated using a. The standard curve will be used in. A standard curve translates absorbance values into concentration. Spectrophotometers are instruments designed to detect the amount of light energy that is absorbed or transmitted by. Spectrophotometry is a method to measure how much a. What Is The Purpose Of A Standard Curve In Spectrophotometry.
From www.slideserve.com
PPT Introduction to Analytical Chemistry PowerPoint Presentation What Is The Purpose Of A Standard Curve In Spectrophotometry Curve of light absorbance and transmission. Standard curves are graphs of light absorbance versus solution concentration which can be used to figure out the solute concentration in unknown samples. See fig 2.1 for an example. Standard curves (also known as calibration curves) represent the relationship between two quantities. A standard curve translates absorbance values into concentration. The standard curve will. What Is The Purpose Of A Standard Curve In Spectrophotometry.
From www.slideserve.com
PPT Spectrophotometry PowerPoint Presentation, free download ID2225954 What Is The Purpose Of A Standard Curve In Spectrophotometry A standard curve translates absorbance values into concentration. Spectrophotometers are instruments designed to detect the amount of light energy that is absorbed or transmitted by. Curve of light absorbance and transmission. Standard curves are graphs of light absorbance versus solution concentration which can be used to figure out the solute concentration in unknown samples. Every standard curve is generated using. What Is The Purpose Of A Standard Curve In Spectrophotometry.
From www.researchgate.net
Standard curve for spectrophotometric determination of cuprous ions What Is The Purpose Of A Standard Curve In Spectrophotometry The standard curve will be used in. Spectrophotometry is a method to measure how much a chemical substance absorbs light by measuring the intensity of light as a beam of light passes through sample solution. Standard curves are graphs of light absorbance versus solution concentration which can be used to figure out the solute concentration in unknown samples. Spectrophotometers are. What Is The Purpose Of A Standard Curve In Spectrophotometry.
From www.researchgate.net
Standard optical density curves versus glucose concentration, measured What Is The Purpose Of A Standard Curve In Spectrophotometry Curve of light absorbance and transmission. See fig 2.1 for an example. Spectrophotometry is a method to measure how much a chemical substance absorbs light by measuring the intensity of light as a beam of light passes through sample solution. The standard curve will be used in. Standard curves (also known as calibration curves) represent the relationship between two quantities.. What Is The Purpose Of A Standard Curve In Spectrophotometry.
From www.nku.edu
Spectrophotometry & Dilutions What Is The Purpose Of A Standard Curve In Spectrophotometry Standard curves (also known as calibration curves) represent the relationship between two quantities. Standard curves are graphs of light absorbance versus solution concentration which can be used to figure out the solute concentration in unknown samples. Spectrophotometry is a method to measure how much a chemical substance absorbs light by measuring the intensity of light as a beam of light. What Is The Purpose Of A Standard Curve In Spectrophotometry.
From studylib.net
Procedure of standard curve What Is The Purpose Of A Standard Curve In Spectrophotometry Every standard curve is generated using a. A standard curve translates absorbance values into concentration. The standard curve will be used in. Standard curves are graphs of light absorbance versus solution concentration which can be used to figure out the solute concentration in unknown samples. Spectrophotometers are instruments designed to detect the amount of light energy that is absorbed or. What Is The Purpose Of A Standard Curve In Spectrophotometry.
From buildingcriteria1.tpub.com
Figure C1. Example standard curve for spectrophotometer. What Is The Purpose Of A Standard Curve In Spectrophotometry A standard curve translates absorbance values into concentration. Curve of light absorbance and transmission. Standard curves are graphs of light absorbance versus solution concentration which can be used to figure out the solute concentration in unknown samples. See fig 2.1 for an example. Every standard curve is generated using a. Spectrophotometers are instruments designed to detect the amount of light. What Is The Purpose Of A Standard Curve In Spectrophotometry.
From www.techwalla.com
How to Plot a Standard Curve in Excel What Is The Purpose Of A Standard Curve In Spectrophotometry See fig 2.1 for an example. Standard curves (also known as calibration curves) represent the relationship between two quantities. Curve of light absorbance and transmission. Spectrophotometry is a method to measure how much a chemical substance absorbs light by measuring the intensity of light as a beam of light passes through sample solution. A standard curve translates absorbance values into. What Is The Purpose Of A Standard Curve In Spectrophotometry.
From exoraqjxh.blob.core.windows.net
Spectrophotometry Graph at Rucker blog What Is The Purpose Of A Standard Curve In Spectrophotometry Standard curves are graphs of light absorbance versus solution concentration which can be used to figure out the solute concentration in unknown samples. Spectrophotometers are instruments designed to detect the amount of light energy that is absorbed or transmitted by. Standard curves (also known as calibration curves) represent the relationship between two quantities. Curve of light absorbance and transmission. See. What Is The Purpose Of A Standard Curve In Spectrophotometry.
From www.researchgate.net
a Spectrum and b standard curve of UVVis spectrophotometer with What Is The Purpose Of A Standard Curve In Spectrophotometry Every standard curve is generated using a. Standard curves are graphs of light absorbance versus solution concentration which can be used to figure out the solute concentration in unknown samples. Curve of light absorbance and transmission. See fig 2.1 for an example. Spectrophotometers are instruments designed to detect the amount of light energy that is absorbed or transmitted by. Standard. What Is The Purpose Of A Standard Curve In Spectrophotometry.
From www.slideserve.com
PPT Spectrophotometry PowerPoint Presentation, free download ID506114 What Is The Purpose Of A Standard Curve In Spectrophotometry Standard curves are graphs of light absorbance versus solution concentration which can be used to figure out the solute concentration in unknown samples. Spectrophotometers are instruments designed to detect the amount of light energy that is absorbed or transmitted by. Curve of light absorbance and transmission. See fig 2.1 for an example. Every standard curve is generated using a. The. What Is The Purpose Of A Standard Curve In Spectrophotometry.
From www.youtube.com
What is a Standard Curve? YouTube What Is The Purpose Of A Standard Curve In Spectrophotometry Spectrophotometry is a method to measure how much a chemical substance absorbs light by measuring the intensity of light as a beam of light passes through sample solution. Spectrophotometers are instruments designed to detect the amount of light energy that is absorbed or transmitted by. Curve of light absorbance and transmission. A standard curve translates absorbance values into concentration. Standard. What Is The Purpose Of A Standard Curve In Spectrophotometry.
From www.slideserve.com
PPT Spectrophotometry PowerPoint Presentation, free download ID506114 What Is The Purpose Of A Standard Curve In Spectrophotometry Standard curves are graphs of light absorbance versus solution concentration which can be used to figure out the solute concentration in unknown samples. Standard curves (also known as calibration curves) represent the relationship between two quantities. Spectrophotometers are instruments designed to detect the amount of light energy that is absorbed or transmitted by. See fig 2.1 for an example. A. What Is The Purpose Of A Standard Curve In Spectrophotometry.
From www.researchgate.net
Standard curve obtained from known concentrations of DOX analyzed by What Is The Purpose Of A Standard Curve In Spectrophotometry Every standard curve is generated using a. Standard curves are graphs of light absorbance versus solution concentration which can be used to figure out the solute concentration in unknown samples. Spectrophotometry is a method to measure how much a chemical substance absorbs light by measuring the intensity of light as a beam of light passes through sample solution. Spectrophotometers are. What Is The Purpose Of A Standard Curve In Spectrophotometry.
From exosnquyu.blob.core.windows.net
How To Make A Standard Curve For Spectrophotometer at Lorraine Hood blog What Is The Purpose Of A Standard Curve In Spectrophotometry The standard curve will be used in. Standard curves (also known as calibration curves) represent the relationship between two quantities. A standard curve translates absorbance values into concentration. Curve of light absorbance and transmission. See fig 2.1 for an example. Standard curves are graphs of light absorbance versus solution concentration which can be used to figure out the solute concentration. What Is The Purpose Of A Standard Curve In Spectrophotometry.
From www.chegg.com
Solved a.) what is the purpose of a standard curve for an What Is The Purpose Of A Standard Curve In Spectrophotometry The standard curve will be used in. Spectrophotometers are instruments designed to detect the amount of light energy that is absorbed or transmitted by. Standard curves (also known as calibration curves) represent the relationship between two quantities. A standard curve translates absorbance values into concentration. Standard curves are graphs of light absorbance versus solution concentration which can be used to. What Is The Purpose Of A Standard Curve In Spectrophotometry.
From ambitiousmares.blogspot.com
32 Label The Graph Below With The Correct Axes For A Standard Curve What Is The Purpose Of A Standard Curve In Spectrophotometry Standard curves (also known as calibration curves) represent the relationship between two quantities. Every standard curve is generated using a. Standard curves are graphs of light absorbance versus solution concentration which can be used to figure out the solute concentration in unknown samples. See fig 2.1 for an example. A standard curve translates absorbance values into concentration. The standard curve. What Is The Purpose Of A Standard Curve In Spectrophotometry.
From www.youtube.com
Lab Review Standard Curve (Unit 2 Spectrophotometry) YouTube What Is The Purpose Of A Standard Curve In Spectrophotometry Spectrophotometry is a method to measure how much a chemical substance absorbs light by measuring the intensity of light as a beam of light passes through sample solution. Every standard curve is generated using a. See fig 2.1 for an example. A standard curve translates absorbance values into concentration. Curve of light absorbance and transmission. Spectrophotometers are instruments designed to. What Is The Purpose Of A Standard Curve In Spectrophotometry.
From www.researchgate.net
An example of a standard curve showing the limits of detection and What Is The Purpose Of A Standard Curve In Spectrophotometry A standard curve translates absorbance values into concentration. See fig 2.1 for an example. Standard curves are graphs of light absorbance versus solution concentration which can be used to figure out the solute concentration in unknown samples. Spectrophotometry is a method to measure how much a chemical substance absorbs light by measuring the intensity of light as a beam of. What Is The Purpose Of A Standard Curve In Spectrophotometry.
From ar.inspiredpencil.com
Spectrophotometer Labeled What Is The Purpose Of A Standard Curve In Spectrophotometry A standard curve translates absorbance values into concentration. Standard curves (also known as calibration curves) represent the relationship between two quantities. See fig 2.1 for an example. Curve of light absorbance and transmission. Spectrophotometry is a method to measure how much a chemical substance absorbs light by measuring the intensity of light as a beam of light passes through sample. What Is The Purpose Of A Standard Curve In Spectrophotometry.
From www.chegg.com
Use The Standard Curve Below To Answer The Followi... What Is The Purpose Of A Standard Curve In Spectrophotometry Spectrophotometers are instruments designed to detect the amount of light energy that is absorbed or transmitted by. Spectrophotometry is a method to measure how much a chemical substance absorbs light by measuring the intensity of light as a beam of light passes through sample solution. The standard curve will be used in. Standard curves are graphs of light absorbance versus. What Is The Purpose Of A Standard Curve In Spectrophotometry.
From www.slideserve.com
PPT Spectrophotometry PowerPoint Presentation, free download ID6911139 What Is The Purpose Of A Standard Curve In Spectrophotometry See fig 2.1 for an example. A standard curve translates absorbance values into concentration. Standard curves are graphs of light absorbance versus solution concentration which can be used to figure out the solute concentration in unknown samples. Curve of light absorbance and transmission. The standard curve will be used in. Spectrophotometers are instruments designed to detect the amount of light. What Is The Purpose Of A Standard Curve In Spectrophotometry.
From www.slideserve.com
PPT Spectrophotometry PowerPoint Presentation, free download ID506114 What Is The Purpose Of A Standard Curve In Spectrophotometry Spectrophotometers are instruments designed to detect the amount of light energy that is absorbed or transmitted by. Standard curves are graphs of light absorbance versus solution concentration which can be used to figure out the solute concentration in unknown samples. Standard curves (also known as calibration curves) represent the relationship between two quantities. Spectrophotometry is a method to measure how. What Is The Purpose Of A Standard Curve In Spectrophotometry.
From www.linquip.com
Beginners Guide What Is a Spectrophotometer Industrial Manufacturing What Is The Purpose Of A Standard Curve In Spectrophotometry Standard curves are graphs of light absorbance versus solution concentration which can be used to figure out the solute concentration in unknown samples. See fig 2.1 for an example. The standard curve will be used in. Spectrophotometers are instruments designed to detect the amount of light energy that is absorbed or transmitted by. Standard curves (also known as calibration curves). What Is The Purpose Of A Standard Curve In Spectrophotometry.
From www.spiedigitallibrary.org
Shedding the light on spectrophotometry the SpecUP educational What Is The Purpose Of A Standard Curve In Spectrophotometry Standard curves (also known as calibration curves) represent the relationship between two quantities. Every standard curve is generated using a. A standard curve translates absorbance values into concentration. Standard curves are graphs of light absorbance versus solution concentration which can be used to figure out the solute concentration in unknown samples. Spectrophotometers are instruments designed to detect the amount of. What Is The Purpose Of A Standard Curve In Spectrophotometry.
From www.researchgate.net
Calibration Curve for the Spectrometer Download Scientific Diagram What Is The Purpose Of A Standard Curve In Spectrophotometry Standard curves are graphs of light absorbance versus solution concentration which can be used to figure out the solute concentration in unknown samples. Every standard curve is generated using a. Standard curves (also known as calibration curves) represent the relationship between two quantities. Curve of light absorbance and transmission. Spectrophotometers are instruments designed to detect the amount of light energy. What Is The Purpose Of A Standard Curve In Spectrophotometry.
From joirwjrbf.blob.core.windows.net
What Are The Main Components Of A Spectrophotometer at Rosa Thompson blog What Is The Purpose Of A Standard Curve In Spectrophotometry The standard curve will be used in. Standard curves are graphs of light absorbance versus solution concentration which can be used to figure out the solute concentration in unknown samples. Every standard curve is generated using a. A standard curve translates absorbance values into concentration. Spectrophotometry is a method to measure how much a chemical substance absorbs light by measuring. What Is The Purpose Of A Standard Curve In Spectrophotometry.
From www.researchgate.net
Standard curve of standard C. oleifera saponins by UV spectrophotometry What Is The Purpose Of A Standard Curve In Spectrophotometry Curve of light absorbance and transmission. Spectrophotometers are instruments designed to detect the amount of light energy that is absorbed or transmitted by. Standard curves (also known as calibration curves) represent the relationship between two quantities. Standard curves are graphs of light absorbance versus solution concentration which can be used to figure out the solute concentration in unknown samples. Spectrophotometry. What Is The Purpose Of A Standard Curve In Spectrophotometry.
From www.researchgate.net
Establishment of the standard curves according to the HPLC and UVVis What Is The Purpose Of A Standard Curve In Spectrophotometry A standard curve translates absorbance values into concentration. Spectrophotometers are instruments designed to detect the amount of light energy that is absorbed or transmitted by. Curve of light absorbance and transmission. Standard curves (also known as calibration curves) represent the relationship between two quantities. Every standard curve is generated using a. Spectrophotometry is a method to measure how much a. What Is The Purpose Of A Standard Curve In Spectrophotometry.