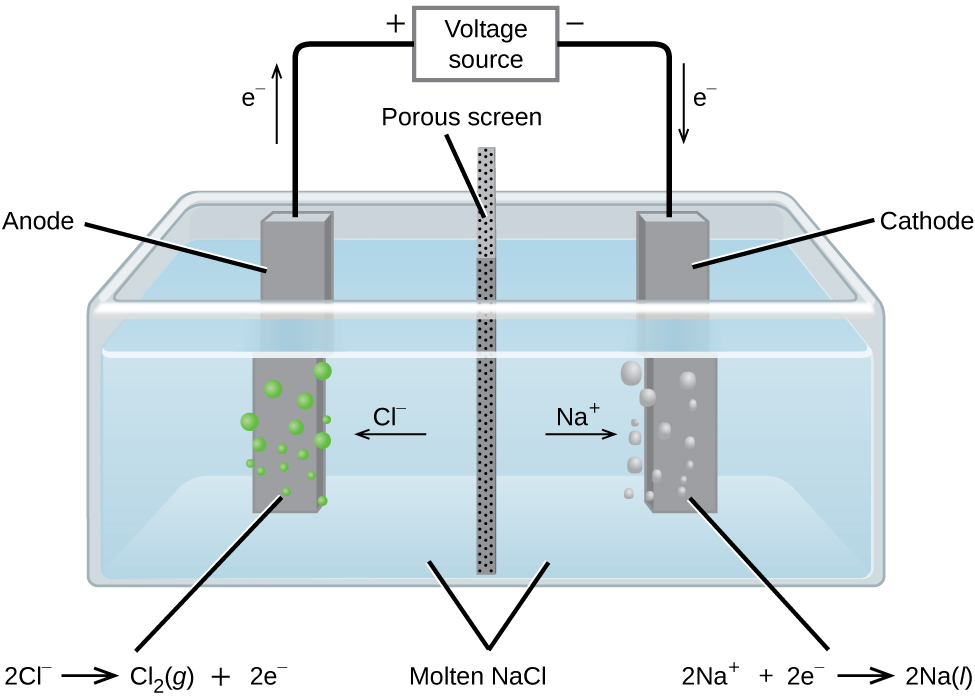
Does Lead Chloride Conduct Electricity . Here, they lose electrons to form chlorine atoms. Lead(ii) chloride is a white solid, melting at 501°c. for example, electrolysing molten lead(ii) bromide gives lead at the cathode: the lead(ii) ions are reduced to lead atoms by gaining electrons. lead(ii) chloride, pbcl 2. Ionic compounds conduct electricity when molten or in. Or if you electrolysed molten sodium chloride: negatively charged chloride ions move to the positive electrode. It is slightly soluble in cold water,. reactive metals are extracted from their ores using electrolysis. Notice that the gain of electrons at.
from chem.libretexts.org
Notice that the gain of electrons at. lead(ii) chloride, pbcl 2. negatively charged chloride ions move to the positive electrode. for example, electrolysing molten lead(ii) bromide gives lead at the cathode: the lead(ii) ions are reduced to lead atoms by gaining electrons. Lead(ii) chloride is a white solid, melting at 501°c. It is slightly soluble in cold water,. Ionic compounds conduct electricity when molten or in. Here, they lose electrons to form chlorine atoms. reactive metals are extracted from their ores using electrolysis.
17.7 Electrolysis Chemistry LibreTexts
Does Lead Chloride Conduct Electricity reactive metals are extracted from their ores using electrolysis. Notice that the gain of electrons at. negatively charged chloride ions move to the positive electrode. Ionic compounds conduct electricity when molten or in. the lead(ii) ions are reduced to lead atoms by gaining electrons. for example, electrolysing molten lead(ii) bromide gives lead at the cathode: lead(ii) chloride, pbcl 2. Here, they lose electrons to form chlorine atoms. Or if you electrolysed molten sodium chloride: Lead(ii) chloride is a white solid, melting at 501°c. It is slightly soluble in cold water,. reactive metals are extracted from their ores using electrolysis.
From spmscience.blog.onlinetuition.com.my
5.5.3 Electrolysis of Molten lead (II) bromide SPM Science Does Lead Chloride Conduct Electricity Here, they lose electrons to form chlorine atoms. reactive metals are extracted from their ores using electrolysis. It is slightly soluble in cold water,. for example, electrolysing molten lead(ii) bromide gives lead at the cathode: lead(ii) chloride, pbcl 2. negatively charged chloride ions move to the positive electrode. the lead(ii) ions are reduced to lead. Does Lead Chloride Conduct Electricity.
From www.linstitute.net
Edexcel IGCSE Chemistry 复习笔记 1.6 5 Ionic compounds Bonds, Structure & Properties翰林国际教育 Does Lead Chloride Conduct Electricity lead(ii) chloride, pbcl 2. negatively charged chloride ions move to the positive electrode. Lead(ii) chloride is a white solid, melting at 501°c. It is slightly soluble in cold water,. reactive metals are extracted from their ores using electrolysis. Ionic compounds conduct electricity when molten or in. for example, electrolysing molten lead(ii) bromide gives lead at the. Does Lead Chloride Conduct Electricity.
From chem.libretexts.org
11.2 Electrolytes Chemistry LibreTexts Does Lead Chloride Conduct Electricity lead(ii) chloride, pbcl 2. Lead(ii) chloride is a white solid, melting at 501°c. Notice that the gain of electrons at. Or if you electrolysed molten sodium chloride: for example, electrolysing molten lead(ii) bromide gives lead at the cathode: Ionic compounds conduct electricity when molten or in. It is slightly soluble in cold water,. negatively charged chloride ions. Does Lead Chloride Conduct Electricity.
From collegedunia.com
Lead II Chloride Formula Properties, Structure, Examples Does Lead Chloride Conduct Electricity Here, they lose electrons to form chlorine atoms. Or if you electrolysed molten sodium chloride: Lead(ii) chloride is a white solid, melting at 501°c. lead(ii) chloride, pbcl 2. reactive metals are extracted from their ores using electrolysis. the lead(ii) ions are reduced to lead atoms by gaining electrons. for example, electrolysing molten lead(ii) bromide gives lead. Does Lead Chloride Conduct Electricity.
From www.911metallurgist.com
Lead Chloride Electrolysis Does Lead Chloride Conduct Electricity Lead(ii) chloride is a white solid, melting at 501°c. the lead(ii) ions are reduced to lead atoms by gaining electrons. Ionic compounds conduct electricity when molten or in. Here, they lose electrons to form chlorine atoms. lead(ii) chloride, pbcl 2. Notice that the gain of electrons at. reactive metals are extracted from their ores using electrolysis. . Does Lead Chloride Conduct Electricity.
From ec.bertabulle.com
How Do Ions Conduct Electricity Does Lead Chloride Conduct Electricity It is slightly soluble in cold water,. the lead(ii) ions are reduced to lead atoms by gaining electrons. Or if you electrolysed molten sodium chloride: for example, electrolysing molten lead(ii) bromide gives lead at the cathode: negatively charged chloride ions move to the positive electrode. Here, they lose electrons to form chlorine atoms. Ionic compounds conduct electricity. Does Lead Chloride Conduct Electricity.
From testbook.com
Lead (II) Chloride Formula Structure, Properties, Uses & More Does Lead Chloride Conduct Electricity Ionic compounds conduct electricity when molten or in. It is slightly soluble in cold water,. the lead(ii) ions are reduced to lead atoms by gaining electrons. Here, they lose electrons to form chlorine atoms. negatively charged chloride ions move to the positive electrode. Notice that the gain of electrons at. reactive metals are extracted from their ores. Does Lead Chloride Conduct Electricity.
From www.chegg.com
Solved Metals, such as sodium, conduct electricity in the Does Lead Chloride Conduct Electricity Lead(ii) chloride is a white solid, melting at 501°c. the lead(ii) ions are reduced to lead atoms by gaining electrons. Or if you electrolysed molten sodium chloride: for example, electrolysing molten lead(ii) bromide gives lead at the cathode: reactive metals are extracted from their ores using electrolysis. Notice that the gain of electrons at. Here, they lose. Does Lead Chloride Conduct Electricity.
From brainly.in
Briefly explain the conductance of electricity by molten sodium chloride without diagram Does Lead Chloride Conduct Electricity Notice that the gain of electrons at. lead(ii) chloride, pbcl 2. reactive metals are extracted from their ores using electrolysis. negatively charged chloride ions move to the positive electrode. Ionic compounds conduct electricity when molten or in. It is slightly soluble in cold water,. the lead(ii) ions are reduced to lead atoms by gaining electrons. Or. Does Lead Chloride Conduct Electricity.
From www.slideserve.com
PPT ELECTROLYSIS A guide for GCSE students PowerPoint Presentation, free download ID297164 Does Lead Chloride Conduct Electricity Notice that the gain of electrons at. Ionic compounds conduct electricity when molten or in. negatively charged chloride ions move to the positive electrode. lead(ii) chloride, pbcl 2. Lead(ii) chloride is a white solid, melting at 501°c. for example, electrolysing molten lead(ii) bromide gives lead at the cathode: reactive metals are extracted from their ores using. Does Lead Chloride Conduct Electricity.
From techiescientist.com
Does Lead Conduct Electricity? Why or Why Not? Techiescientist Does Lead Chloride Conduct Electricity Here, they lose electrons to form chlorine atoms. negatively charged chloride ions move to the positive electrode. reactive metals are extracted from their ores using electrolysis. Notice that the gain of electrons at. It is slightly soluble in cold water,. the lead(ii) ions are reduced to lead atoms by gaining electrons. lead(ii) chloride, pbcl 2. . Does Lead Chloride Conduct Electricity.
From www.thoughtco.com
Electrical Conductivity of Metals Does Lead Chloride Conduct Electricity Here, they lose electrons to form chlorine atoms. Or if you electrolysed molten sodium chloride: for example, electrolysing molten lead(ii) bromide gives lead at the cathode: It is slightly soluble in cold water,. reactive metals are extracted from their ores using electrolysis. the lead(ii) ions are reduced to lead atoms by gaining electrons. negatively charged chloride. Does Lead Chloride Conduct Electricity.
From electricity.selotips.com
How Do Ions Conduct Electricity Does Lead Chloride Conduct Electricity the lead(ii) ions are reduced to lead atoms by gaining electrons. It is slightly soluble in cold water,. lead(ii) chloride, pbcl 2. Notice that the gain of electrons at. Or if you electrolysed molten sodium chloride: reactive metals are extracted from their ores using electrolysis. negatively charged chloride ions move to the positive electrode. Here, they. Does Lead Chloride Conduct Electricity.
From www.slideserve.com
PPT Electrolysis PowerPoint Presentation, free download ID5813936 Does Lead Chloride Conduct Electricity reactive metals are extracted from their ores using electrolysis. Here, they lose electrons to form chlorine atoms. for example, electrolysing molten lead(ii) bromide gives lead at the cathode: Ionic compounds conduct electricity when molten or in. Or if you electrolysed molten sodium chloride: Lead(ii) chloride is a white solid, melting at 501°c. It is slightly soluble in cold. Does Lead Chloride Conduct Electricity.
From exovzbqxo.blob.core.windows.net
Are Ionic Compounds Conductors Of Electricity at Paul Dejesus blog Does Lead Chloride Conduct Electricity It is slightly soluble in cold water,. reactive metals are extracted from their ores using electrolysis. Here, they lose electrons to form chlorine atoms. negatively charged chloride ions move to the positive electrode. Notice that the gain of electrons at. Or if you electrolysed molten sodium chloride: Lead(ii) chloride is a white solid, melting at 501°c. the. Does Lead Chloride Conduct Electricity.
From www.teachoo.com
The table shown below gives information about four Chemistry MCQ Does Lead Chloride Conduct Electricity Here, they lose electrons to form chlorine atoms. Ionic compounds conduct electricity when molten or in. reactive metals are extracted from their ores using electrolysis. the lead(ii) ions are reduced to lead atoms by gaining electrons. for example, electrolysing molten lead(ii) bromide gives lead at the cathode: It is slightly soluble in cold water,. Or if you. Does Lead Chloride Conduct Electricity.
From byjus.com
Give reasons for the following(a) Solid sodium chloride does not conduct electricity while Does Lead Chloride Conduct Electricity Lead(ii) chloride is a white solid, melting at 501°c. reactive metals are extracted from their ores using electrolysis. the lead(ii) ions are reduced to lead atoms by gaining electrons. negatively charged chloride ions move to the positive electrode. Or if you electrolysed molten sodium chloride: lead(ii) chloride, pbcl 2. Notice that the gain of electrons at.. Does Lead Chloride Conduct Electricity.
From slideplayer.com
Chapter 5 Introduction to Reactions in Aqueous Solutions. ppt download Does Lead Chloride Conduct Electricity Notice that the gain of electrons at. Lead(ii) chloride is a white solid, melting at 501°c. Or if you electrolysed molten sodium chloride: Here, they lose electrons to form chlorine atoms. It is slightly soluble in cold water,. the lead(ii) ions are reduced to lead atoms by gaining electrons. lead(ii) chloride, pbcl 2. for example, electrolysing molten. Does Lead Chloride Conduct Electricity.
From slideplayer.com
Ionic Compounds Chapter ppt download Does Lead Chloride Conduct Electricity for example, electrolysing molten lead(ii) bromide gives lead at the cathode: Ionic compounds conduct electricity when molten or in. It is slightly soluble in cold water,. Here, they lose electrons to form chlorine atoms. lead(ii) chloride, pbcl 2. negatively charged chloride ions move to the positive electrode. the lead(ii) ions are reduced to lead atoms by. Does Lead Chloride Conduct Electricity.
From es.slideshare.net
Chemical bonding Does Lead Chloride Conduct Electricity negatively charged chloride ions move to the positive electrode. Or if you electrolysed molten sodium chloride: reactive metals are extracted from their ores using electrolysis. It is slightly soluble in cold water,. Ionic compounds conduct electricity when molten or in. Lead(ii) chloride is a white solid, melting at 501°c. lead(ii) chloride, pbcl 2. the lead(ii) ions. Does Lead Chloride Conduct Electricity.
From www.slideserve.com
PPT Electrolysis L.O. I can explain why different substances appear at the anode and cathode Does Lead Chloride Conduct Electricity Lead(ii) chloride is a white solid, melting at 501°c. the lead(ii) ions are reduced to lead atoms by gaining electrons. Notice that the gain of electrons at. for example, electrolysing molten lead(ii) bromide gives lead at the cathode: Ionic compounds conduct electricity when molten or in. negatively charged chloride ions move to the positive electrode. lead(ii). Does Lead Chloride Conduct Electricity.
From www.slideserve.com
PPT Unit 4, Lesson 2 PowerPoint Presentation, free download ID3037878 Does Lead Chloride Conduct Electricity the lead(ii) ions are reduced to lead atoms by gaining electrons. lead(ii) chloride, pbcl 2. Lead(ii) chloride is a white solid, melting at 501°c. Or if you electrolysed molten sodium chloride: It is slightly soluble in cold water,. negatively charged chloride ions move to the positive electrode. Here, they lose electrons to form chlorine atoms. for. Does Lead Chloride Conduct Electricity.
From chem.libretexts.org
17.7 Electrolysis Chemistry LibreTexts Does Lead Chloride Conduct Electricity Ionic compounds conduct electricity when molten or in. Or if you electrolysed molten sodium chloride: negatively charged chloride ions move to the positive electrode. Lead(ii) chloride is a white solid, melting at 501°c. for example, electrolysing molten lead(ii) bromide gives lead at the cathode: the lead(ii) ions are reduced to lead atoms by gaining electrons. reactive. Does Lead Chloride Conduct Electricity.
From www.slideshare.net
Chemical bonding Does Lead Chloride Conduct Electricity Or if you electrolysed molten sodium chloride: the lead(ii) ions are reduced to lead atoms by gaining electrons. It is slightly soluble in cold water,. Notice that the gain of electrons at. Here, they lose electrons to form chlorine atoms. Ionic compounds conduct electricity when molten or in. negatively charged chloride ions move to the positive electrode. . Does Lead Chloride Conduct Electricity.
From chemwiki.ucdavis.edu
Electrolytic Cells Chemwiki Does Lead Chloride Conduct Electricity reactive metals are extracted from their ores using electrolysis. Ionic compounds conduct electricity when molten or in. the lead(ii) ions are reduced to lead atoms by gaining electrons. lead(ii) chloride, pbcl 2. Notice that the gain of electrons at. It is slightly soluble in cold water,. for example, electrolysing molten lead(ii) bromide gives lead at the. Does Lead Chloride Conduct Electricity.
From www.slideserve.com
PPT Periodicity (AHL) PowerPoint Presentation, free download ID2249355 Does Lead Chloride Conduct Electricity Notice that the gain of electrons at. Ionic compounds conduct electricity when molten or in. negatively charged chloride ions move to the positive electrode. lead(ii) chloride, pbcl 2. reactive metals are extracted from their ores using electrolysis. Or if you electrolysed molten sodium chloride: It is slightly soluble in cold water,. Lead(ii) chloride is a white solid,. Does Lead Chloride Conduct Electricity.
From www.911metallurgist.com
Lead Chloride Electrolysis Does Lead Chloride Conduct Electricity Notice that the gain of electrons at. It is slightly soluble in cold water,. Ionic compounds conduct electricity when molten or in. Or if you electrolysed molten sodium chloride: Here, they lose electrons to form chlorine atoms. lead(ii) chloride, pbcl 2. negatively charged chloride ions move to the positive electrode. reactive metals are extracted from their ores. Does Lead Chloride Conduct Electricity.
From fphoto.photoshelter.com
science chemistry conductivity test Fundamental Photographs The Art of Science Does Lead Chloride Conduct Electricity lead(ii) chloride, pbcl 2. Ionic compounds conduct electricity when molten or in. the lead(ii) ions are reduced to lead atoms by gaining electrons. Or if you electrolysed molten sodium chloride: negatively charged chloride ions move to the positive electrode. It is slightly soluble in cold water,. for example, electrolysing molten lead(ii) bromide gives lead at the. Does Lead Chloride Conduct Electricity.
From vdocuments.mx
Electrolysis of Lead Bromide and Brine [PPTX Powerpoint] Does Lead Chloride Conduct Electricity Notice that the gain of electrons at. Or if you electrolysed molten sodium chloride: for example, electrolysing molten lead(ii) bromide gives lead at the cathode: lead(ii) chloride, pbcl 2. Lead(ii) chloride is a white solid, melting at 501°c. negatively charged chloride ions move to the positive electrode. reactive metals are extracted from their ores using electrolysis.. Does Lead Chloride Conduct Electricity.
From www.slideshare.net
Chem matters ch6_ionic_bond Does Lead Chloride Conduct Electricity the lead(ii) ions are reduced to lead atoms by gaining electrons. It is slightly soluble in cold water,. Notice that the gain of electrons at. lead(ii) chloride, pbcl 2. Ionic compounds conduct electricity when molten or in. reactive metals are extracted from their ores using electrolysis. for example, electrolysing molten lead(ii) bromide gives lead at the. Does Lead Chloride Conduct Electricity.
From www.911metallurgist.com
Lead Chloride Electrolysis Does Lead Chloride Conduct Electricity for example, electrolysing molten lead(ii) bromide gives lead at the cathode: lead(ii) chloride, pbcl 2. It is slightly soluble in cold water,. Here, they lose electrons to form chlorine atoms. the lead(ii) ions are reduced to lead atoms by gaining electrons. Ionic compounds conduct electricity when molten or in. Lead(ii) chloride is a white solid, melting at. Does Lead Chloride Conduct Electricity.
From www.youtube.com
How Ionic Compounds Conduct Electricity GCSE Chemistry YouTube Does Lead Chloride Conduct Electricity for example, electrolysing molten lead(ii) bromide gives lead at the cathode: It is slightly soluble in cold water,. Ionic compounds conduct electricity when molten or in. negatively charged chloride ions move to the positive electrode. Or if you electrolysed molten sodium chloride: Notice that the gain of electrons at. Here, they lose electrons to form chlorine atoms. . Does Lead Chloride Conduct Electricity.
From dxoqdjcqk.blob.core.windows.net
Lead Chloride Formula at Joesph Hernandez blog Does Lead Chloride Conduct Electricity reactive metals are extracted from their ores using electrolysis. lead(ii) chloride, pbcl 2. It is slightly soluble in cold water,. Notice that the gain of electrons at. Here, they lose electrons to form chlorine atoms. for example, electrolysing molten lead(ii) bromide gives lead at the cathode: Or if you electrolysed molten sodium chloride: Lead(ii) chloride is a. Does Lead Chloride Conduct Electricity.
From www.shalom-education.com
The Basics of Electrolysis GCSE Chemistry Revision Does Lead Chloride Conduct Electricity Or if you electrolysed molten sodium chloride: negatively charged chloride ions move to the positive electrode. the lead(ii) ions are reduced to lead atoms by gaining electrons. lead(ii) chloride, pbcl 2. It is slightly soluble in cold water,. Ionic compounds conduct electricity when molten or in. Lead(ii) chloride is a white solid, melting at 501°c. Notice that. Does Lead Chloride Conduct Electricity.
From www.slideserve.com
PPT What are bonds? PowerPoint Presentation, free download ID5980343 Does Lead Chloride Conduct Electricity the lead(ii) ions are reduced to lead atoms by gaining electrons. It is slightly soluble in cold water,. reactive metals are extracted from their ores using electrolysis. lead(ii) chloride, pbcl 2. Notice that the gain of electrons at. Ionic compounds conduct electricity when molten or in. for example, electrolysing molten lead(ii) bromide gives lead at the. Does Lead Chloride Conduct Electricity.