Zinc Gluconate Solubility In Water . Solubility table for water at temperature. Dissolve 0.5 g in a mixture of 2 ml of hydrochloric acid (~330 g/l) ts and 10 ml of water r. Zinc gluconate is a zinc salt of gluconic acid comprised of two gluconic acid molecules for each zinc cation (2+). Water temperature can have a significant effect on the solubility of compounds. Assay— dissolve about 700 mg of zinc gluconate, accurately weighed, in 100 ml of water. 98.0 per cent to 102.0 per cent (anhydrous substance). Zinc oxide (zno), very often used in topical products, is sparingly soluble in water, with a solubility. Boil for 5 minutes, allow to cool, add 10. The table below provides information on the variation of solubility of different substances (mostly inorganic.
from www.chegg.com
Dissolve 0.5 g in a mixture of 2 ml of hydrochloric acid (~330 g/l) ts and 10 ml of water r. Zinc oxide (zno), very often used in topical products, is sparingly soluble in water, with a solubility. Boil for 5 minutes, allow to cool, add 10. Water temperature can have a significant effect on the solubility of compounds. 98.0 per cent to 102.0 per cent (anhydrous substance). Zinc gluconate is a zinc salt of gluconic acid comprised of two gluconic acid molecules for each zinc cation (2+). Solubility table for water at temperature. The table below provides information on the variation of solubility of different substances (mostly inorganic. Assay— dissolve about 700 mg of zinc gluconate, accurately weighed, in 100 ml of water.
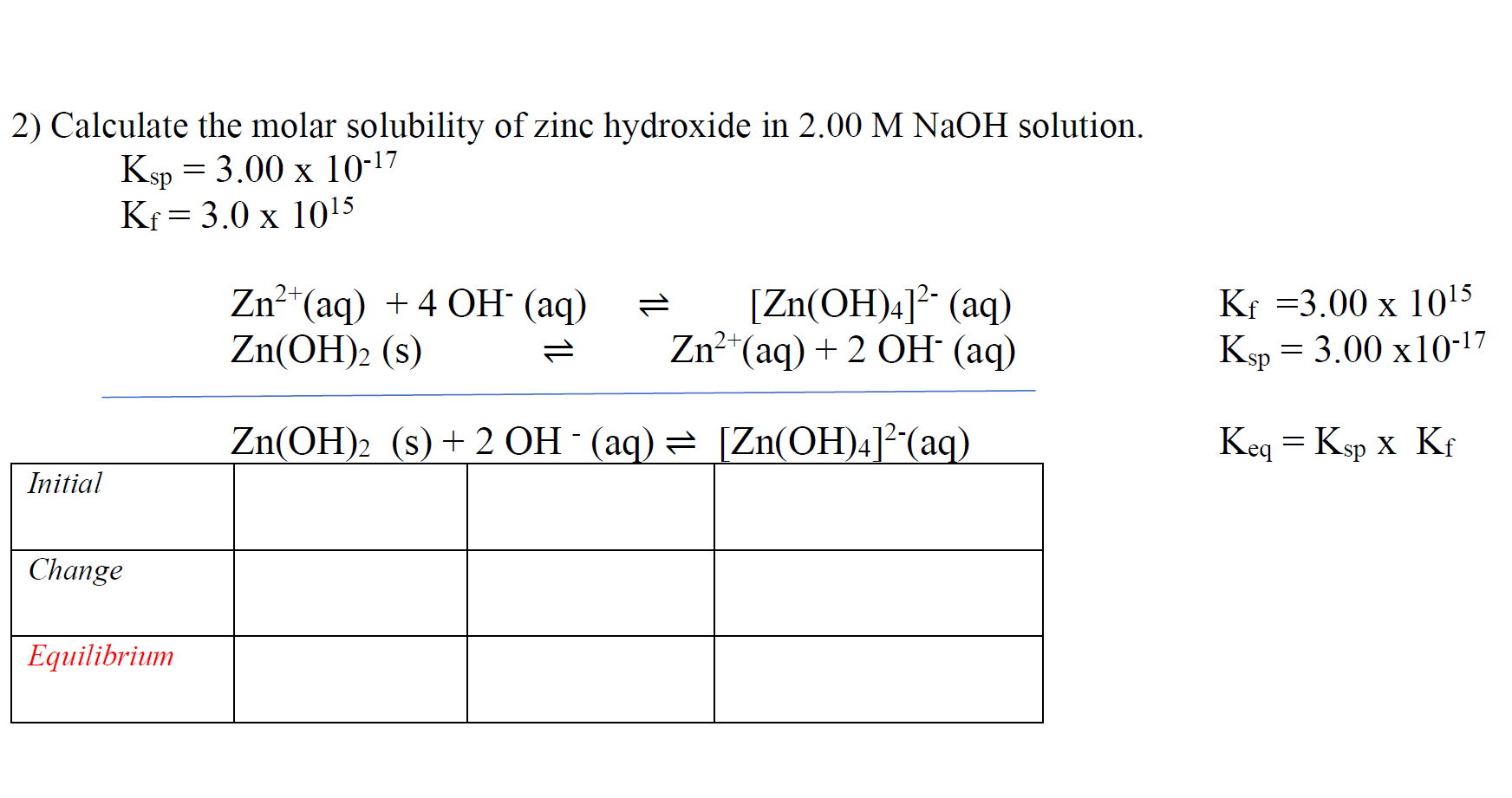
Solved 2) Calculate the molar solubility of zinc hydroxide
Zinc Gluconate Solubility In Water Dissolve 0.5 g in a mixture of 2 ml of hydrochloric acid (~330 g/l) ts and 10 ml of water r. Solubility table for water at temperature. Zinc gluconate is a zinc salt of gluconic acid comprised of two gluconic acid molecules for each zinc cation (2+). Dissolve 0.5 g in a mixture of 2 ml of hydrochloric acid (~330 g/l) ts and 10 ml of water r. Zinc oxide (zno), very often used in topical products, is sparingly soluble in water, with a solubility. Assay— dissolve about 700 mg of zinc gluconate, accurately weighed, in 100 ml of water. Water temperature can have a significant effect on the solubility of compounds. The table below provides information on the variation of solubility of different substances (mostly inorganic. Boil for 5 minutes, allow to cool, add 10. 98.0 per cent to 102.0 per cent (anhydrous substance).
From www.researchgate.net
What is the proper way to prepare a zinc chloride containing buffer for Zinc Gluconate Solubility In Water Zinc gluconate is a zinc salt of gluconic acid comprised of two gluconic acid molecules for each zinc cation (2+). Assay— dissolve about 700 mg of zinc gluconate, accurately weighed, in 100 ml of water. Dissolve 0.5 g in a mixture of 2 ml of hydrochloric acid (~330 g/l) ts and 10 ml of water r. Water temperature can have. Zinc Gluconate Solubility In Water.
From www.youtube.com
Zinc Powder in water YouTube Zinc Gluconate Solubility In Water Zinc oxide (zno), very often used in topical products, is sparingly soluble in water, with a solubility. Water temperature can have a significant effect on the solubility of compounds. The table below provides information on the variation of solubility of different substances (mostly inorganic. Solubility table for water at temperature. Boil for 5 minutes, allow to cool, add 10. Zinc. Zinc Gluconate Solubility In Water.
From www.researchgate.net
Zinc solubility stability of LDRLEzinc chelate, zinc sulphate and zinc Zinc Gluconate Solubility In Water Solubility table for water at temperature. Dissolve 0.5 g in a mixture of 2 ml of hydrochloric acid (~330 g/l) ts and 10 ml of water r. Assay— dissolve about 700 mg of zinc gluconate, accurately weighed, in 100 ml of water. Water temperature can have a significant effect on the solubility of compounds. The table below provides information on. Zinc Gluconate Solubility In Water.
From www.chegg.com
Solved Compare the solubility of zinc sulfide in each of the Zinc Gluconate Solubility In Water 98.0 per cent to 102.0 per cent (anhydrous substance). Boil for 5 minutes, allow to cool, add 10. Dissolve 0.5 g in a mixture of 2 ml of hydrochloric acid (~330 g/l) ts and 10 ml of water r. Zinc oxide (zno), very often used in topical products, is sparingly soluble in water, with a solubility. Solubility table for water. Zinc Gluconate Solubility In Water.
From www.youtube.com
Zinc metal reaction Nitric acid reaction Zinc in nitric acid Zinc Gluconate Solubility In Water Assay— dissolve about 700 mg of zinc gluconate, accurately weighed, in 100 ml of water. Solubility table for water at temperature. 98.0 per cent to 102.0 per cent (anhydrous substance). The table below provides information on the variation of solubility of different substances (mostly inorganic. Dissolve 0.5 g in a mixture of 2 ml of hydrochloric acid (~330 g/l) ts. Zinc Gluconate Solubility In Water.
From www.ahabiochem.com
China Low Price Zinc Gluconate CAS 4468024 Manufacturers, Suppliers Zinc Gluconate Solubility In Water Assay— dissolve about 700 mg of zinc gluconate, accurately weighed, in 100 ml of water. Water temperature can have a significant effect on the solubility of compounds. Zinc gluconate is a zinc salt of gluconic acid comprised of two gluconic acid molecules for each zinc cation (2+). Zinc oxide (zno), very often used in topical products, is sparingly soluble in. Zinc Gluconate Solubility In Water.
From edurev.in
The reaction of zinc with dilute and concentrated nitric acid Zinc Gluconate Solubility In Water Dissolve 0.5 g in a mixture of 2 ml of hydrochloric acid (~330 g/l) ts and 10 ml of water r. Assay— dissolve about 700 mg of zinc gluconate, accurately weighed, in 100 ml of water. Water temperature can have a significant effect on the solubility of compounds. Solubility table for water at temperature. 98.0 per cent to 102.0 per. Zinc Gluconate Solubility In Water.
From agutsygirl.com
Zinc Bisglycinate vs Zinc Gluconate (A Guide to All Things Zinc) A Zinc Gluconate Solubility In Water Zinc oxide (zno), very often used in topical products, is sparingly soluble in water, with a solubility. Zinc gluconate is a zinc salt of gluconic acid comprised of two gluconic acid molecules for each zinc cation (2+). Water temperature can have a significant effect on the solubility of compounds. Assay— dissolve about 700 mg of zinc gluconate, accurately weighed, in. Zinc Gluconate Solubility In Water.
From www.researchgate.net
Zinc solubility stability of LDRLEzinc chelate, zinc sulphate and zinc Zinc Gluconate Solubility In Water Dissolve 0.5 g in a mixture of 2 ml of hydrochloric acid (~330 g/l) ts and 10 ml of water r. The table below provides information on the variation of solubility of different substances (mostly inorganic. Zinc oxide (zno), very often used in topical products, is sparingly soluble in water, with a solubility. Zinc gluconate is a zinc salt of. Zinc Gluconate Solubility In Water.
From www.indiamart.com
Indian Zinc Gluconate Powder, 25 Kg, Prescription at Rs 300/box in Mumbai Zinc Gluconate Solubility In Water Dissolve 0.5 g in a mixture of 2 ml of hydrochloric acid (~330 g/l) ts and 10 ml of water r. Assay— dissolve about 700 mg of zinc gluconate, accurately weighed, in 100 ml of water. 98.0 per cent to 102.0 per cent (anhydrous substance). Zinc gluconate is a zinc salt of gluconic acid comprised of two gluconic acid molecules. Zinc Gluconate Solubility In Water.
From www.chegg.com
Solved Compare the solubility of zinc carbonate in each of Zinc Gluconate Solubility In Water 98.0 per cent to 102.0 per cent (anhydrous substance). Dissolve 0.5 g in a mixture of 2 ml of hydrochloric acid (~330 g/l) ts and 10 ml of water r. Boil for 5 minutes, allow to cool, add 10. Zinc oxide (zno), very often used in topical products, is sparingly soluble in water, with a solubility. The table below provides. Zinc Gluconate Solubility In Water.
From www.semanticscholar.org
Figure 1 from COMPARISON OF SOLUBILITY OF ZINC PHOSPHATE AND GLASS Zinc Gluconate Solubility In Water Assay— dissolve about 700 mg of zinc gluconate, accurately weighed, in 100 ml of water. Dissolve 0.5 g in a mixture of 2 ml of hydrochloric acid (~330 g/l) ts and 10 ml of water r. The table below provides information on the variation of solubility of different substances (mostly inorganic. Water temperature can have a significant effect on the. Zinc Gluconate Solubility In Water.
From lifepathdoc.com
Pyrithione Zinc vs Selenium Sulfide Which is Better? Zinc Gluconate Solubility In Water 98.0 per cent to 102.0 per cent (anhydrous substance). Boil for 5 minutes, allow to cool, add 10. Water temperature can have a significant effect on the solubility of compounds. Zinc oxide (zno), very often used in topical products, is sparingly soluble in water, with a solubility. Dissolve 0.5 g in a mixture of 2 ml of hydrochloric acid (~330. Zinc Gluconate Solubility In Water.
From www.chegg.com
Solved Compare the solubility of zinc sulfide in each of the Zinc Gluconate Solubility In Water 98.0 per cent to 102.0 per cent (anhydrous substance). Assay— dissolve about 700 mg of zinc gluconate, accurately weighed, in 100 ml of water. Zinc gluconate is a zinc salt of gluconic acid comprised of two gluconic acid molecules for each zinc cation (2+). Solubility table for water at temperature. Zinc oxide (zno), very often used in topical products, is. Zinc Gluconate Solubility In Water.
From www.chegg.com
Solved 2) Calculate the molar solubility of zinc hydroxide Zinc Gluconate Solubility In Water Boil for 5 minutes, allow to cool, add 10. Zinc gluconate is a zinc salt of gluconic acid comprised of two gluconic acid molecules for each zinc cation (2+). 98.0 per cent to 102.0 per cent (anhydrous substance). Water temperature can have a significant effect on the solubility of compounds. Assay— dissolve about 700 mg of zinc gluconate, accurately weighed,. Zinc Gluconate Solubility In Water.
From www.researchgate.net
Calcium gluconate monohydrate and Form I solubility curve. Download Zinc Gluconate Solubility In Water Zinc oxide (zno), very often used in topical products, is sparingly soluble in water, with a solubility. 98.0 per cent to 102.0 per cent (anhydrous substance). Assay— dissolve about 700 mg of zinc gluconate, accurately weighed, in 100 ml of water. Solubility table for water at temperature. Water temperature can have a significant effect on the solubility of compounds. Boil. Zinc Gluconate Solubility In Water.
From differencebtw.com
Zinc Citrate vs. Zinc Gluconate Know the Difference Zinc Gluconate Solubility In Water Dissolve 0.5 g in a mixture of 2 ml of hydrochloric acid (~330 g/l) ts and 10 ml of water r. Water temperature can have a significant effect on the solubility of compounds. Solubility table for water at temperature. Zinc gluconate is a zinc salt of gluconic acid comprised of two gluconic acid molecules for each zinc cation (2+). Boil. Zinc Gluconate Solubility In Water.
From www.sxytbio.com
Good Price Zinc Gluconate Powder Manufacturers Factory Zinc Gluconate Solubility In Water Zinc oxide (zno), very often used in topical products, is sparingly soluble in water, with a solubility. Dissolve 0.5 g in a mixture of 2 ml of hydrochloric acid (~330 g/l) ts and 10 ml of water r. Solubility table for water at temperature. Assay— dissolve about 700 mg of zinc gluconate, accurately weighed, in 100 ml of water. Boil. Zinc Gluconate Solubility In Water.
From www.researchgate.net
Zinc solubility stability of LDRLEzinc chelate, zinc sulphate and zinc Zinc Gluconate Solubility In Water Water temperature can have a significant effect on the solubility of compounds. Solubility table for water at temperature. Assay— dissolve about 700 mg of zinc gluconate, accurately weighed, in 100 ml of water. Zinc gluconate is a zinc salt of gluconic acid comprised of two gluconic acid molecules for each zinc cation (2+). 98.0 per cent to 102.0 per cent. Zinc Gluconate Solubility In Water.
From www.chegg.com
Solved (a) From the solubility product of zinc ferrocyanide, Zinc Gluconate Solubility In Water Zinc gluconate is a zinc salt of gluconic acid comprised of two gluconic acid molecules for each zinc cation (2+). Boil for 5 minutes, allow to cool, add 10. Dissolve 0.5 g in a mixture of 2 ml of hydrochloric acid (~330 g/l) ts and 10 ml of water r. Water temperature can have a significant effect on the solubility. Zinc Gluconate Solubility In Water.
From advancedchemsys.com
Zinc Removal From Water Advanced Chemical Systems Zinc Gluconate Solubility In Water Assay— dissolve about 700 mg of zinc gluconate, accurately weighed, in 100 ml of water. Solubility table for water at temperature. The table below provides information on the variation of solubility of different substances (mostly inorganic. Water temperature can have a significant effect on the solubility of compounds. Zinc gluconate is a zinc salt of gluconic acid comprised of two. Zinc Gluconate Solubility In Water.
From calebcroomphysci4dummies.weebly.com
Solubility Physical Science For Dummies Zinc Gluconate Solubility In Water Assay— dissolve about 700 mg of zinc gluconate, accurately weighed, in 100 ml of water. Zinc gluconate is a zinc salt of gluconic acid comprised of two gluconic acid molecules for each zinc cation (2+). Water temperature can have a significant effect on the solubility of compounds. Boil for 5 minutes, allow to cool, add 10. The table below provides. Zinc Gluconate Solubility In Water.
From www.mdpi.com
Foods Free FullText Study on the In Silico Screening and Zinc Gluconate Solubility In Water 98.0 per cent to 102.0 per cent (anhydrous substance). Assay— dissolve about 700 mg of zinc gluconate, accurately weighed, in 100 ml of water. Zinc gluconate is a zinc salt of gluconic acid comprised of two gluconic acid molecules for each zinc cation (2+). Solubility table for water at temperature. Zinc oxide (zno), very often used in topical products, is. Zinc Gluconate Solubility In Water.
From intershop.com.vn
ZINC GLUCONATE Zinc Gluconate Solubility In Water Zinc gluconate is a zinc salt of gluconic acid comprised of two gluconic acid molecules for each zinc cation (2+). Zinc oxide (zno), very often used in topical products, is sparingly soluble in water, with a solubility. Assay— dissolve about 700 mg of zinc gluconate, accurately weighed, in 100 ml of water. 98.0 per cent to 102.0 per cent (anhydrous. Zinc Gluconate Solubility In Water.
From www.slideserve.com
PPT Solubility Notes PowerPoint Presentation, free download ID5606676 Zinc Gluconate Solubility In Water Assay— dissolve about 700 mg of zinc gluconate, accurately weighed, in 100 ml of water. Dissolve 0.5 g in a mixture of 2 ml of hydrochloric acid (~330 g/l) ts and 10 ml of water r. Solubility table for water at temperature. 98.0 per cent to 102.0 per cent (anhydrous substance). Water temperature can have a significant effect on the. Zinc Gluconate Solubility In Water.
From glchems.en.made-in-china.com
Cheap and Fine Chemical CAS 4468024 Zinc Gluconate China Health Zinc Gluconate Solubility In Water Solubility table for water at temperature. Boil for 5 minutes, allow to cool, add 10. Zinc oxide (zno), very often used in topical products, is sparingly soluble in water, with a solubility. The table below provides information on the variation of solubility of different substances (mostly inorganic. 98.0 per cent to 102.0 per cent (anhydrous substance). Water temperature can have. Zinc Gluconate Solubility In Water.
From www.researchgate.net
2) gives the solubility of magnesium in water at 25°C as a function of Zinc Gluconate Solubility In Water Boil for 5 minutes, allow to cool, add 10. Water temperature can have a significant effect on the solubility of compounds. Zinc gluconate is a zinc salt of gluconic acid comprised of two gluconic acid molecules for each zinc cation (2+). Dissolve 0.5 g in a mixture of 2 ml of hydrochloric acid (~330 g/l) ts and 10 ml of. Zinc Gluconate Solubility In Water.
From www.researchgate.net
Zinc solubility stability of LDRLEzinc chelate, zinc sulphate and zinc Zinc Gluconate Solubility In Water Water temperature can have a significant effect on the solubility of compounds. Boil for 5 minutes, allow to cool, add 10. Zinc gluconate is a zinc salt of gluconic acid comprised of two gluconic acid molecules for each zinc cation (2+). The table below provides information on the variation of solubility of different substances (mostly inorganic. Zinc oxide (zno), very. Zinc Gluconate Solubility In Water.
From www.researchgate.net
Effects of added glycine, alanine and serine on zinc citrate solubility Zinc Gluconate Solubility In Water Solubility table for water at temperature. Water temperature can have a significant effect on the solubility of compounds. Zinc oxide (zno), very often used in topical products, is sparingly soluble in water, with a solubility. Boil for 5 minutes, allow to cool, add 10. Assay— dissolve about 700 mg of zinc gluconate, accurately weighed, in 100 ml of water. Zinc. Zinc Gluconate Solubility In Water.
From healthplus.com.bd
NOW Supplements Zinc Gluconate 50mg in Bangladesh, 100 Tabs Zinc Gluconate Solubility In Water Boil for 5 minutes, allow to cool, add 10. 98.0 per cent to 102.0 per cent (anhydrous substance). Zinc oxide (zno), very often used in topical products, is sparingly soluble in water, with a solubility. Solubility table for water at temperature. Zinc gluconate is a zinc salt of gluconic acid comprised of two gluconic acid molecules for each zinc cation. Zinc Gluconate Solubility In Water.
From www.studocu.com
Solubility Table CHM 110 CHEM135F23 September 13 Solubility Table Zinc Gluconate Solubility In Water Boil for 5 minutes, allow to cool, add 10. Zinc gluconate is a zinc salt of gluconic acid comprised of two gluconic acid molecules for each zinc cation (2+). Dissolve 0.5 g in a mixture of 2 ml of hydrochloric acid (~330 g/l) ts and 10 ml of water r. Water temperature can have a significant effect on the solubility. Zinc Gluconate Solubility In Water.
From www.researchgate.net
The solubility of trisodium phosphate in hightemperature water as a Zinc Gluconate Solubility In Water Water temperature can have a significant effect on the solubility of compounds. 98.0 per cent to 102.0 per cent (anhydrous substance). The table below provides information on the variation of solubility of different substances (mostly inorganic. Solubility table for water at temperature. Zinc oxide (zno), very often used in topical products, is sparingly soluble in water, with a solubility. Assay—. Zinc Gluconate Solubility In Water.
From socratic.org
How does zinc form an insoluble zinc hydroxide in water? Socratic Zinc Gluconate Solubility In Water Dissolve 0.5 g in a mixture of 2 ml of hydrochloric acid (~330 g/l) ts and 10 ml of water r. Zinc oxide (zno), very often used in topical products, is sparingly soluble in water, with a solubility. Zinc gluconate is a zinc salt of gluconic acid comprised of two gluconic acid molecules for each zinc cation (2+). Water temperature. Zinc Gluconate Solubility In Water.
From www.slideserve.com
PPT Chapter 10 Structure and Synthesis of Alcohols PowerPoint Zinc Gluconate Solubility In Water Zinc gluconate is a zinc salt of gluconic acid comprised of two gluconic acid molecules for each zinc cation (2+). Boil for 5 minutes, allow to cool, add 10. Zinc oxide (zno), very often used in topical products, is sparingly soluble in water, with a solubility. 98.0 per cent to 102.0 per cent (anhydrous substance). Dissolve 0.5 g in a. Zinc Gluconate Solubility In Water.
From www.researchgate.net
Calcium gluconate monohydrate and Form I solubility curve. Download Zinc Gluconate Solubility In Water The table below provides information on the variation of solubility of different substances (mostly inorganic. Solubility table for water at temperature. Zinc gluconate is a zinc salt of gluconic acid comprised of two gluconic acid molecules for each zinc cation (2+). 98.0 per cent to 102.0 per cent (anhydrous substance). Water temperature can have a significant effect on the solubility. Zinc Gluconate Solubility In Water.