Is Table Salt Acidic Or Basic . hydrochloric acid, or hcl, for example, reacts with sodium hydroxide, or naoh, to produce sodium chloride, nacl, also. it could contain either an excess of hydronium ions or an excess of hydroxide ions because the nature of the salt formed. Learn how to measure ph and see the ph values of. since acids and bases can be weak or strong there are four types of salts that can result, and these will result in neutral,. when is a salt solution basic or acidic? dissolving table salt in water does not change its ph, so ordinary salt water is neutral. a salt may be defined as the product of a neutralization reaction of an acid and a base. There are several guiding principles that summarize the outcome: The prototype “salt,” of course, is sodium. table salt is neither acidic nor alkaline;
from www.teachoo.com
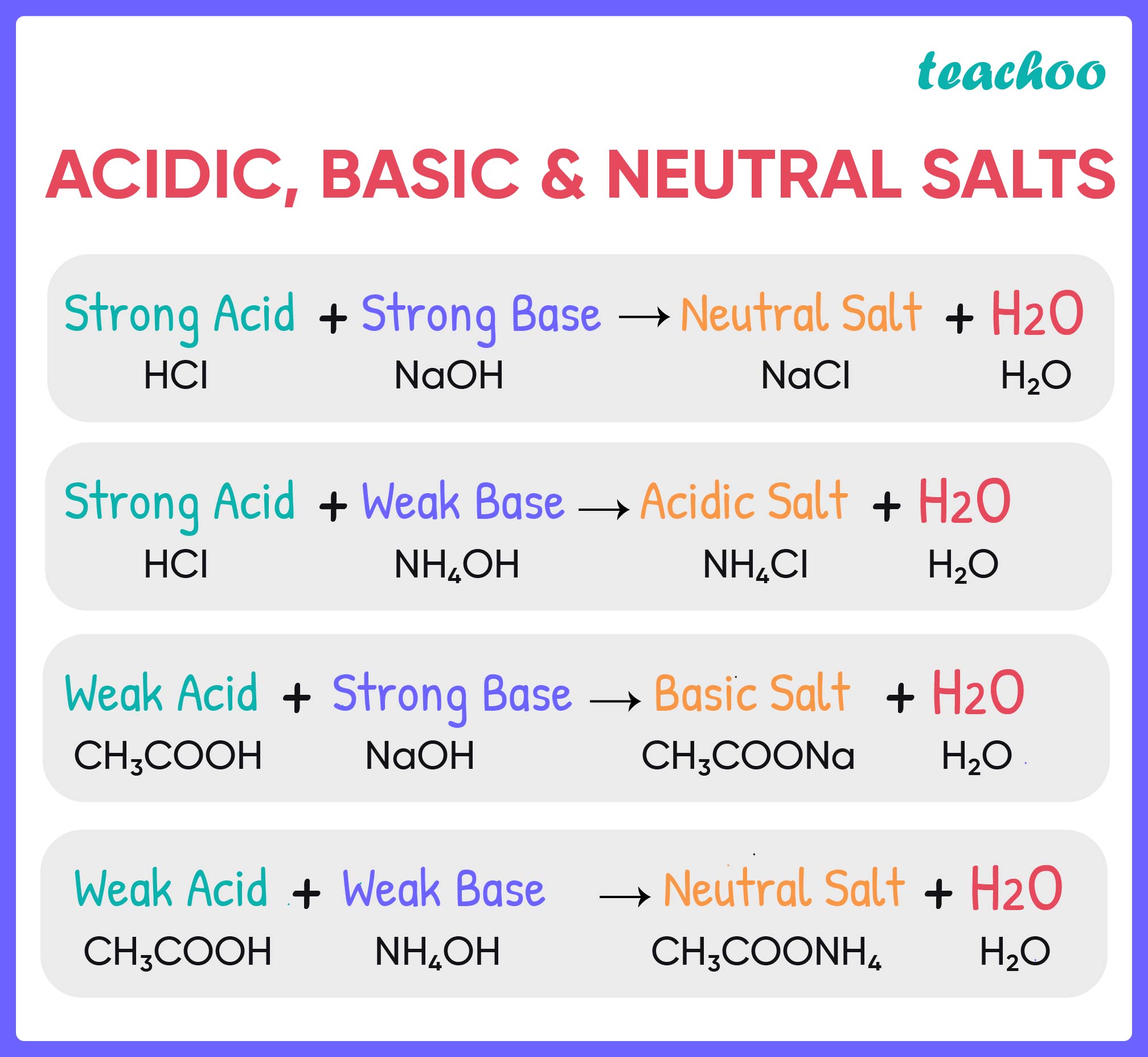
since acids and bases can be weak or strong there are four types of salts that can result, and these will result in neutral,. The prototype “salt,” of course, is sodium. table salt is neither acidic nor alkaline; it could contain either an excess of hydronium ions or an excess of hydroxide ions because the nature of the salt formed. when is a salt solution basic or acidic? a salt may be defined as the product of a neutralization reaction of an acid and a base. dissolving table salt in water does not change its ph, so ordinary salt water is neutral. hydrochloric acid, or hcl, for example, reacts with sodium hydroxide, or naoh, to produce sodium chloride, nacl, also. There are several guiding principles that summarize the outcome: Learn how to measure ph and see the ph values of.
Salts and it's Properties (with Examples) Acids, Bases and Salt
Is Table Salt Acidic Or Basic table salt is neither acidic nor alkaline; hydrochloric acid, or hcl, for example, reacts with sodium hydroxide, or naoh, to produce sodium chloride, nacl, also. There are several guiding principles that summarize the outcome: a salt may be defined as the product of a neutralization reaction of an acid and a base. since acids and bases can be weak or strong there are four types of salts that can result, and these will result in neutral,. dissolving table salt in water does not change its ph, so ordinary salt water is neutral. Learn how to measure ph and see the ph values of. table salt is neither acidic nor alkaline; The prototype “salt,” of course, is sodium. when is a salt solution basic or acidic? it could contain either an excess of hydronium ions or an excess of hydroxide ions because the nature of the salt formed.
From www.thoughtco.com
Chemical Composition of Table Salt Is Table Salt Acidic Or Basic There are several guiding principles that summarize the outcome: The prototype “salt,” of course, is sodium. hydrochloric acid, or hcl, for example, reacts with sodium hydroxide, or naoh, to produce sodium chloride, nacl, also. dissolving table salt in water does not change its ph, so ordinary salt water is neutral. Learn how to measure ph and see the. Is Table Salt Acidic Or Basic.
From www.slideserve.com
PPT Unit 6 Chpt 14&15 Acid/Base PowerPoint Presentation, free Is Table Salt Acidic Or Basic The prototype “salt,” of course, is sodium. when is a salt solution basic or acidic? it could contain either an excess of hydronium ions or an excess of hydroxide ions because the nature of the salt formed. table salt is neither acidic nor alkaline; Learn how to measure ph and see the ph values of. hydrochloric. Is Table Salt Acidic Or Basic.
From worksheetlistfy.z19.web.core.windows.net
Acids Bases And Salts Ph Scale Is Table Salt Acidic Or Basic table salt is neither acidic nor alkaline; a salt may be defined as the product of a neutralization reaction of an acid and a base. hydrochloric acid, or hcl, for example, reacts with sodium hydroxide, or naoh, to produce sodium chloride, nacl, also. when is a salt solution basic or acidic? since acids and bases. Is Table Salt Acidic Or Basic.
From www.differencebetween.com
Difference Between Acidic Salt and Basic Salt Compare the Difference Is Table Salt Acidic Or Basic it could contain either an excess of hydronium ions or an excess of hydroxide ions because the nature of the salt formed. table salt is neither acidic nor alkaline; when is a salt solution basic or acidic? Learn how to measure ph and see the ph values of. There are several guiding principles that summarize the outcome:. Is Table Salt Acidic Or Basic.
From sciencenotes.org
The pH Scale of Common Chemicals Is Table Salt Acidic Or Basic hydrochloric acid, or hcl, for example, reacts with sodium hydroxide, or naoh, to produce sodium chloride, nacl, also. table salt is neither acidic nor alkaline; There are several guiding principles that summarize the outcome: Learn how to measure ph and see the ph values of. when is a salt solution basic or acidic? dissolving table salt. Is Table Salt Acidic Or Basic.
From www.teachoo.com
MCQ Identify the basic salt from the following salts (a) Na2CO3 Is Table Salt Acidic Or Basic dissolving table salt in water does not change its ph, so ordinary salt water is neutral. hydrochloric acid, or hcl, for example, reacts with sodium hydroxide, or naoh, to produce sodium chloride, nacl, also. since acids and bases can be weak or strong there are four types of salts that can result, and these will result in. Is Table Salt Acidic Or Basic.
From z-cm.blogspot.com
Is Table Salt Acidic Basic Or Neutral Decoration Examples Is Table Salt Acidic Or Basic dissolving table salt in water does not change its ph, so ordinary salt water is neutral. table salt is neither acidic nor alkaline; There are several guiding principles that summarize the outcome: it could contain either an excess of hydronium ions or an excess of hydroxide ions because the nature of the salt formed. since acids. Is Table Salt Acidic Or Basic.
From www.youtube.com
Classifying salt solution as acidic, basic, or neutral YouTube Is Table Salt Acidic Or Basic dissolving table salt in water does not change its ph, so ordinary salt water is neutral. a salt may be defined as the product of a neutralization reaction of an acid and a base. There are several guiding principles that summarize the outcome: Learn how to measure ph and see the ph values of. when is a. Is Table Salt Acidic Or Basic.
From www.fity.club
Sat Chemistry Chemical Formulas Names And Formulas Of Is Table Salt Acidic Or Basic Learn how to measure ph and see the ph values of. The prototype “salt,” of course, is sodium. a salt may be defined as the product of a neutralization reaction of an acid and a base. when is a salt solution basic or acidic? dissolving table salt in water does not change its ph, so ordinary salt. Is Table Salt Acidic Or Basic.
From z-cm.blogspot.com
Is Table Salt Acidic Basic Or Neutral Decoration Examples Is Table Salt Acidic Or Basic Learn how to measure ph and see the ph values of. There are several guiding principles that summarize the outcome: hydrochloric acid, or hcl, for example, reacts with sodium hydroxide, or naoh, to produce sodium chloride, nacl, also. dissolving table salt in water does not change its ph, so ordinary salt water is neutral. table salt is. Is Table Salt Acidic Or Basic.
From z-cm.blogspot.com
Is Table Salt Acidic Basic Or Neutral Decoration Examples Is Table Salt Acidic Or Basic it could contain either an excess of hydronium ions or an excess of hydroxide ions because the nature of the salt formed. a salt may be defined as the product of a neutralization reaction of an acid and a base. Learn how to measure ph and see the ph values of. since acids and bases can be. Is Table Salt Acidic Or Basic.
From arielle-well-wolfe.blogspot.com
Chemistry Form 4 Chapter 7 Acid and Base Is Table Salt Acidic Or Basic a salt may be defined as the product of a neutralization reaction of an acid and a base. There are several guiding principles that summarize the outcome: it could contain either an excess of hydronium ions or an excess of hydroxide ions because the nature of the salt formed. The prototype “salt,” of course, is sodium. dissolving. Is Table Salt Acidic Or Basic.
From www.slideserve.com
PPT Acids, Bases, and SALTS PowerPoint Presentation, free download Is Table Salt Acidic Or Basic The prototype “salt,” of course, is sodium. when is a salt solution basic or acidic? a salt may be defined as the product of a neutralization reaction of an acid and a base. since acids and bases can be weak or strong there are four types of salts that can result, and these will result in neutral,.. Is Table Salt Acidic Or Basic.
From alevelchemistry.co.uk
Acids Facts, Summary, Weak & Strong ALevel Chemistry Revision Is Table Salt Acidic Or Basic The prototype “salt,” of course, is sodium. dissolving table salt in water does not change its ph, so ordinary salt water is neutral. it could contain either an excess of hydronium ions or an excess of hydroxide ions because the nature of the salt formed. when is a salt solution basic or acidic? hydrochloric acid, or. Is Table Salt Acidic Or Basic.
From www.sliderbase.com
AcidBase Properties of Salts Presentation Chemistry Is Table Salt Acidic Or Basic The prototype “salt,” of course, is sodium. when is a salt solution basic or acidic? since acids and bases can be weak or strong there are four types of salts that can result, and these will result in neutral,. Learn how to measure ph and see the ph values of. There are several guiding principles that summarize the. Is Table Salt Acidic Or Basic.
From dddiqyzoeco.blob.core.windows.net
Table Salt Acidic Or Alkaline at Derek Stokes blog Is Table Salt Acidic Or Basic when is a salt solution basic or acidic? Learn how to measure ph and see the ph values of. hydrochloric acid, or hcl, for example, reacts with sodium hydroxide, or naoh, to produce sodium chloride, nacl, also. it could contain either an excess of hydronium ions or an excess of hydroxide ions because the nature of the. Is Table Salt Acidic Or Basic.
From www.vrogue.co
Acids Bases And Salts Igcse Chemistry Vrogue Is Table Salt Acidic Or Basic since acids and bases can be weak or strong there are four types of salts that can result, and these will result in neutral,. it could contain either an excess of hydronium ions or an excess of hydroxide ions because the nature of the salt formed. Learn how to measure ph and see the ph values of. The. Is Table Salt Acidic Or Basic.
From www.wipeoutreflux.com
Is Salt Acidic or Alkaline? (table and sea salt covered) Is Table Salt Acidic Or Basic Learn how to measure ph and see the ph values of. The prototype “salt,” of course, is sodium. dissolving table salt in water does not change its ph, so ordinary salt water is neutral. a salt may be defined as the product of a neutralization reaction of an acid and a base. since acids and bases can. Is Table Salt Acidic Or Basic.
From z-cm.blogspot.com
Is Table Salt Acidic Basic Or Neutral Decoration Examples Is Table Salt Acidic Or Basic The prototype “salt,” of course, is sodium. dissolving table salt in water does not change its ph, so ordinary salt water is neutral. since acids and bases can be weak or strong there are four types of salts that can result, and these will result in neutral,. Learn how to measure ph and see the ph values of.. Is Table Salt Acidic Or Basic.
From www.slideserve.com
PPT Unit 6 Chpt 14&15 Acid/Base PowerPoint Presentation, free Is Table Salt Acidic Or Basic a salt may be defined as the product of a neutralization reaction of an acid and a base. table salt is neither acidic nor alkaline; it could contain either an excess of hydronium ions or an excess of hydroxide ions because the nature of the salt formed. when is a salt solution basic or acidic? . Is Table Salt Acidic Or Basic.
From z-cm.blogspot.com
Is Table Salt Acidic Basic Or Neutral Decoration Examples Is Table Salt Acidic Or Basic when is a salt solution basic or acidic? it could contain either an excess of hydronium ions or an excess of hydroxide ions because the nature of the salt formed. table salt is neither acidic nor alkaline; hydrochloric acid, or hcl, for example, reacts with sodium hydroxide, or naoh, to produce sodium chloride, nacl, also. There. Is Table Salt Acidic Or Basic.
From www.sigmaaldrich.cn
Acid and Base Chart — Table of Acids & Bases Is Table Salt Acidic Or Basic since acids and bases can be weak or strong there are four types of salts that can result, and these will result in neutral,. dissolving table salt in water does not change its ph, so ordinary salt water is neutral. hydrochloric acid, or hcl, for example, reacts with sodium hydroxide, or naoh, to produce sodium chloride, nacl,. Is Table Salt Acidic Or Basic.
From www.youtube.com
Types of Salts, Normal salt, Acidic salt, Basic salt, Double salt Is Table Salt Acidic Or Basic Learn how to measure ph and see the ph values of. The prototype “salt,” of course, is sodium. it could contain either an excess of hydronium ions or an excess of hydroxide ions because the nature of the salt formed. a salt may be defined as the product of a neutralization reaction of an acid and a base.. Is Table Salt Acidic Or Basic.
From dddiqyzoeco.blob.core.windows.net
Table Salt Acidic Or Alkaline at Derek Stokes blog Is Table Salt Acidic Or Basic when is a salt solution basic or acidic? There are several guiding principles that summarize the outcome: The prototype “salt,” of course, is sodium. since acids and bases can be weak or strong there are four types of salts that can result, and these will result in neutral,. a salt may be defined as the product of. Is Table Salt Acidic Or Basic.
From www.slideserve.com
PPT Acids produce H + in solution, example HCl Bases produce OH in Is Table Salt Acidic Or Basic since acids and bases can be weak or strong there are four types of salts that can result, and these will result in neutral,. dissolving table salt in water does not change its ph, so ordinary salt water is neutral. Learn how to measure ph and see the ph values of. table salt is neither acidic nor. Is Table Salt Acidic Or Basic.
From www.teachoo.com
Salts and it's Properties (with Examples) Acids, Bases and Salt Is Table Salt Acidic Or Basic The prototype “salt,” of course, is sodium. since acids and bases can be weak or strong there are four types of salts that can result, and these will result in neutral,. it could contain either an excess of hydronium ions or an excess of hydroxide ions because the nature of the salt formed. hydrochloric acid, or hcl,. Is Table Salt Acidic Or Basic.
From www.youtube.com
Is Table Salt (NaCl ) Ionic or Covalent/Molecular? YouTube Is Table Salt Acidic Or Basic a salt may be defined as the product of a neutralization reaction of an acid and a base. dissolving table salt in water does not change its ph, so ordinary salt water is neutral. table salt is neither acidic nor alkaline; The prototype “salt,” of course, is sodium. hydrochloric acid, or hcl, for example, reacts with. Is Table Salt Acidic Or Basic.
From www.youtube.com
Acidbase properties of salts Acids and bases AP Chemistry Khan Is Table Salt Acidic Or Basic table salt is neither acidic nor alkaline; a salt may be defined as the product of a neutralization reaction of an acid and a base. The prototype “salt,” of course, is sodium. hydrochloric acid, or hcl, for example, reacts with sodium hydroxide, or naoh, to produce sodium chloride, nacl, also. dissolving table salt in water does. Is Table Salt Acidic Or Basic.
From z-cm.blogspot.com
Is Table Salt Acidic Basic Or Neutral Decoration Examples Is Table Salt Acidic Or Basic since acids and bases can be weak or strong there are four types of salts that can result, and these will result in neutral,. dissolving table salt in water does not change its ph, so ordinary salt water is neutral. a salt may be defined as the product of a neutralization reaction of an acid and a. Is Table Salt Acidic Or Basic.
From www.youtube.com
Classify each salt as acidic, basic, or neutral YouTube Is Table Salt Acidic Or Basic when is a salt solution basic or acidic? There are several guiding principles that summarize the outcome: dissolving table salt in water does not change its ph, so ordinary salt water is neutral. a salt may be defined as the product of a neutralization reaction of an acid and a base. The prototype “salt,” of course, is. Is Table Salt Acidic Or Basic.
From www.slideserve.com
PPT Unit 18 AcidBase Equilibria Buffers & Hydrolysis PowerPoint Is Table Salt Acidic Or Basic dissolving table salt in water does not change its ph, so ordinary salt water is neutral. a salt may be defined as the product of a neutralization reaction of an acid and a base. hydrochloric acid, or hcl, for example, reacts with sodium hydroxide, or naoh, to produce sodium chloride, nacl, also. Learn how to measure ph. Is Table Salt Acidic Or Basic.
From mavink.com
Basic Acidic Chart Is Table Salt Acidic Or Basic There are several guiding principles that summarize the outcome: hydrochloric acid, or hcl, for example, reacts with sodium hydroxide, or naoh, to produce sodium chloride, nacl, also. it could contain either an excess of hydronium ions or an excess of hydroxide ions because the nature of the salt formed. dissolving table salt in water does not change. Is Table Salt Acidic Or Basic.
From www.youtube.com
Acidic, Basic, and Neutral Salts Ionic Compounds YouTube Is Table Salt Acidic Or Basic The prototype “salt,” of course, is sodium. dissolving table salt in water does not change its ph, so ordinary salt water is neutral. since acids and bases can be weak or strong there are four types of salts that can result, and these will result in neutral,. when is a salt solution basic or acidic? There are. Is Table Salt Acidic Or Basic.
From yuliana-blogmoran.blogspot.com
How to Know if a Salt Is Acidic or Basic Is Table Salt Acidic Or Basic hydrochloric acid, or hcl, for example, reacts with sodium hydroxide, or naoh, to produce sodium chloride, nacl, also. it could contain either an excess of hydronium ions or an excess of hydroxide ions because the nature of the salt formed. table salt is neither acidic nor alkaline; a salt may be defined as the product of. Is Table Salt Acidic Or Basic.
From general.chemistrysteps.com
Acidity of a Salt Solution Chemistry Steps Is Table Salt Acidic Or Basic it could contain either an excess of hydronium ions or an excess of hydroxide ions because the nature of the salt formed. a salt may be defined as the product of a neutralization reaction of an acid and a base. dissolving table salt in water does not change its ph, so ordinary salt water is neutral. There. Is Table Salt Acidic Or Basic.