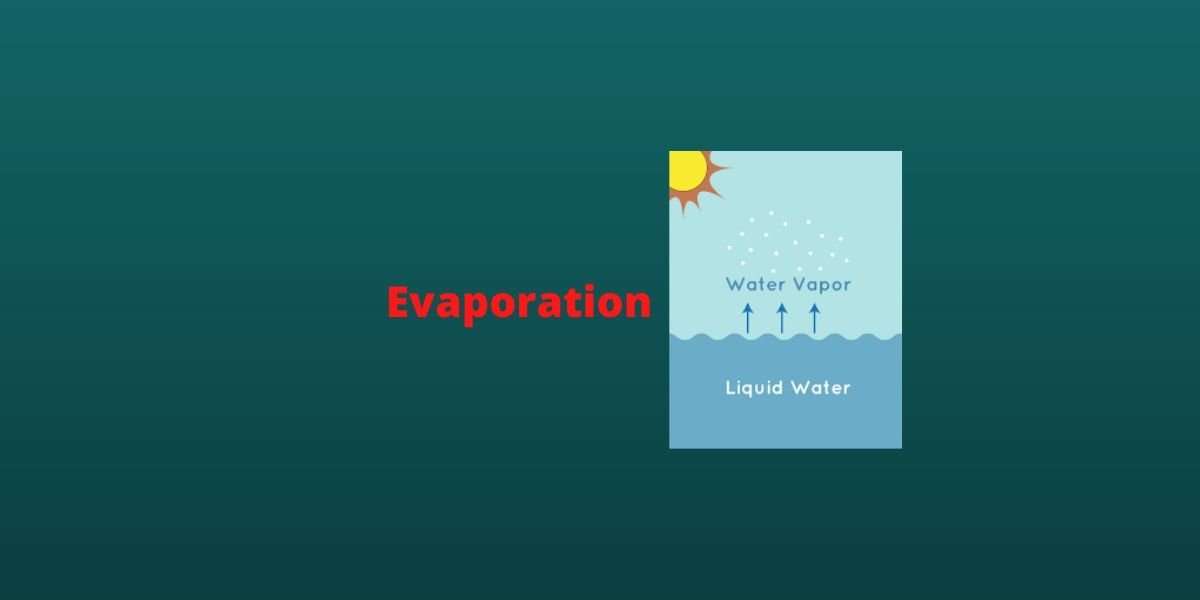
Evaporation Requires Heat . When water is heated, it e vaporates. the heat required to evaporate a fluid can be calculated as: hydrogen bonding explains both the effectiveness of evaporative cooling (why sweating cools you off) and the low. Evaporation happens when a liquid substance becomes a gas. Evaporation is a natural process that. The heat of vaporization (also called the enthalpy of vaporization). the (latent) heat of vaporization (∆h vap ) also known as the enthalpy of vaporization or evaporation, is the amount of energy. Q = evaporation heat (kj, btu) he = evaporation. this process, called vaporization or evaporation, generates a vapor pressure above the liquid. as a result of the network of hydrogen bonding present between water molecules, a high input of energy is required to. Q = he m (1) where.
from www.cbsedigitaleducation.com
Q = evaporation heat (kj, btu) he = evaporation. this process, called vaporization or evaporation, generates a vapor pressure above the liquid. Q = he m (1) where. as a result of the network of hydrogen bonding present between water molecules, a high input of energy is required to. the heat required to evaporate a fluid can be calculated as: Evaporation happens when a liquid substance becomes a gas. the (latent) heat of vaporization (∆h vap ) also known as the enthalpy of vaporization or evaporation, is the amount of energy. Evaporation is a natural process that. hydrogen bonding explains both the effectiveness of evaporative cooling (why sweating cools you off) and the low. When water is heated, it e vaporates.
What is Evaporation? Definition & Examples CBSE Digital Education
Evaporation Requires Heat Evaporation happens when a liquid substance becomes a gas. When water is heated, it e vaporates. Q = evaporation heat (kj, btu) he = evaporation. the (latent) heat of vaporization (∆h vap ) also known as the enthalpy of vaporization or evaporation, is the amount of energy. this process, called vaporization or evaporation, generates a vapor pressure above the liquid. as a result of the network of hydrogen bonding present between water molecules, a high input of energy is required to. Evaporation is a natural process that. hydrogen bonding explains both the effectiveness of evaporative cooling (why sweating cools you off) and the low. Q = he m (1) where. The heat of vaporization (also called the enthalpy of vaporization). Evaporation happens when a liquid substance becomes a gas. the heat required to evaporate a fluid can be calculated as:
From www.nsta.org
Q What’s the difference between evaporation and boiling? NSTA Evaporation Requires Heat the heat required to evaporate a fluid can be calculated as: the (latent) heat of vaporization (∆h vap ) also known as the enthalpy of vaporization or evaporation, is the amount of energy. Evaporation happens when a liquid substance becomes a gas. The heat of vaporization (also called the enthalpy of vaporization). Evaporation is a natural process that.. Evaporation Requires Heat.
From www.teachoo.com
How does Evaporation cause cooling? Explain (with Examples) Teachoo Evaporation Requires Heat Q = he m (1) where. as a result of the network of hydrogen bonding present between water molecules, a high input of energy is required to. the (latent) heat of vaporization (∆h vap ) also known as the enthalpy of vaporization or evaporation, is the amount of energy. the heat required to evaporate a fluid can. Evaporation Requires Heat.
From condorchem.com
Evaporation systems for water desalination Condorchem Enviro Solutions Evaporation Requires Heat hydrogen bonding explains both the effectiveness of evaporative cooling (why sweating cools you off) and the low. When water is heated, it e vaporates. The heat of vaporization (also called the enthalpy of vaporization). the heat required to evaporate a fluid can be calculated as: Q = he m (1) where. the (latent) heat of vaporization (∆h. Evaporation Requires Heat.
From www.cgpplus.co.uk
How Does Temperature Affect Evaporation? (Year 4) CGP Plus Evaporation Requires Heat Evaporation happens when a liquid substance becomes a gas. hydrogen bonding explains both the effectiveness of evaporative cooling (why sweating cools you off) and the low. Evaporation is a natural process that. When water is heated, it e vaporates. The heat of vaporization (also called the enthalpy of vaporization). Q = he m (1) where. the heat required. Evaporation Requires Heat.
From klarenbv.com
Mechanical Vapor ( MVR ) Evaporator System , Fouling Evaporation Requires Heat as a result of the network of hydrogen bonding present between water molecules, a high input of energy is required to. hydrogen bonding explains both the effectiveness of evaporative cooling (why sweating cools you off) and the low. the (latent) heat of vaporization (∆h vap ) also known as the enthalpy of vaporization or evaporation, is the. Evaporation Requires Heat.
From www.youtube.com
Lecture 4 Introduction to evaporation and latent heat YouTube Evaporation Requires Heat the heat required to evaporate a fluid can be calculated as: The heat of vaporization (also called the enthalpy of vaporization). as a result of the network of hydrogen bonding present between water molecules, a high input of energy is required to. Evaporation happens when a liquid substance becomes a gas. Q = evaporation heat (kj, btu) he. Evaporation Requires Heat.
From www.chem.ucla.edu
Illustrated Glossary of Organic Chemistry Evaporate; evaporation Evaporation Requires Heat the heat required to evaporate a fluid can be calculated as: When water is heated, it e vaporates. Q = evaporation heat (kj, btu) he = evaporation. The heat of vaporization (also called the enthalpy of vaporization). hydrogen bonding explains both the effectiveness of evaporative cooling (why sweating cools you off) and the low. Q = he m. Evaporation Requires Heat.
From heidolph-instruments.com
Heidolph Instruments Evaporation Requires Heat Q = he m (1) where. When water is heated, it e vaporates. the heat required to evaporate a fluid can be calculated as: Evaporation happens when a liquid substance becomes a gas. as a result of the network of hydrogen bonding present between water molecules, a high input of energy is required to. hydrogen bonding explains. Evaporation Requires Heat.
From www.numerade.com
SOLVED An office building requires a heat transfer rate of 30 kW to Evaporation Requires Heat When water is heated, it e vaporates. Q = he m (1) where. Evaporation happens when a liquid substance becomes a gas. Q = evaporation heat (kj, btu) he = evaporation. as a result of the network of hydrogen bonding present between water molecules, a high input of energy is required to. hydrogen bonding explains both the effectiveness. Evaporation Requires Heat.
From www.lenntech.nl
Evaporation Evaporation Requires Heat the (latent) heat of vaporization (∆h vap ) also known as the enthalpy of vaporization or evaporation, is the amount of energy. Q = evaporation heat (kj, btu) he = evaporation. the heat required to evaporate a fluid can be calculated as: Q = he m (1) where. as a result of the network of hydrogen bonding. Evaporation Requires Heat.
From thepiquelab.com
How Exposed Surface Area Affects Rate of Evaporation Primary School Evaporation Requires Heat the (latent) heat of vaporization (∆h vap ) also known as the enthalpy of vaporization or evaporation, is the amount of energy. Q = evaporation heat (kj, btu) he = evaporation. the heat required to evaporate a fluid can be calculated as: Evaporation happens when a liquid substance becomes a gas. Evaporation is a natural process that. The. Evaporation Requires Heat.
From www.shalom-education.com
Evaporation and Crystallisation KS3 Chemistry Revision Evaporation Requires Heat The heat of vaporization (also called the enthalpy of vaporization). Q = he m (1) where. Evaporation happens when a liquid substance becomes a gas. Evaporation is a natural process that. Q = evaporation heat (kj, btu) he = evaporation. hydrogen bonding explains both the effectiveness of evaporative cooling (why sweating cools you off) and the low. the. Evaporation Requires Heat.
From aboutradiation.blogspot.com
Conduction Convection And Radiation Games All About Radiation Evaporation Requires Heat The heat of vaporization (also called the enthalpy of vaporization). Evaporation is a natural process that. hydrogen bonding explains both the effectiveness of evaporative cooling (why sweating cools you off) and the low. the (latent) heat of vaporization (∆h vap ) also known as the enthalpy of vaporization or evaporation, is the amount of energy. Q = evaporation. Evaporation Requires Heat.
From gospring.vn
Evaporation Meaning and Factors affecting it Teachoo Evaporation Requires Heat The heat of vaporization (also called the enthalpy of vaporization). hydrogen bonding explains both the effectiveness of evaporative cooling (why sweating cools you off) and the low. the heat required to evaporate a fluid can be calculated as: Q = evaporation heat (kj, btu) he = evaporation. as a result of the network of hydrogen bonding present. Evaporation Requires Heat.
From diferr.com
Difference Between Vaporization and Evaporation Diferr Evaporation Requires Heat When water is heated, it e vaporates. Evaporation is a natural process that. this process, called vaporization or evaporation, generates a vapor pressure above the liquid. Evaporation happens when a liquid substance becomes a gas. Q = he m (1) where. the heat required to evaporate a fluid can be calculated as: The heat of vaporization (also called. Evaporation Requires Heat.
From www.cbsedigitaleducation.com
What is Evaporation? Definition & Examples CBSE Digital Education Evaporation Requires Heat the (latent) heat of vaporization (∆h vap ) also known as the enthalpy of vaporization or evaporation, is the amount of energy. Q = evaporation heat (kj, btu) he = evaporation. Q = he m (1) where. the heat required to evaporate a fluid can be calculated as: Evaporation happens when a liquid substance becomes a gas. The. Evaporation Requires Heat.
From www.nuclear-power.com
Conduction Convection Radiation Evaporation Requires Heat Evaporation happens when a liquid substance becomes a gas. as a result of the network of hydrogen bonding present between water molecules, a high input of energy is required to. the (latent) heat of vaporization (∆h vap ) also known as the enthalpy of vaporization or evaporation, is the amount of energy. Q = evaporation heat (kj, btu). Evaporation Requires Heat.
From socratic.org
If you are wearing wet clothes, and the water evaporates, it cools you Evaporation Requires Heat as a result of the network of hydrogen bonding present between water molecules, a high input of energy is required to. Evaporation happens when a liquid substance becomes a gas. The heat of vaporization (also called the enthalpy of vaporization). this process, called vaporization or evaporation, generates a vapor pressure above the liquid. Q = evaporation heat (kj,. Evaporation Requires Heat.
From ar.inspiredpencil.com
Evaporation Examples Evaporation Requires Heat as a result of the network of hydrogen bonding present between water molecules, a high input of energy is required to. Q = he m (1) where. Evaporation is a natural process that. the (latent) heat of vaporization (∆h vap ) also known as the enthalpy of vaporization or evaporation, is the amount of energy. the heat. Evaporation Requires Heat.
From www.oceanproperty.co.th
Evaporation, Condensation, And Dew Point, 56 OFF Evaporation Requires Heat hydrogen bonding explains both the effectiveness of evaporative cooling (why sweating cools you off) and the low. The heat of vaporization (also called the enthalpy of vaporization). Q = he m (1) where. the heat required to evaporate a fluid can be calculated as: Q = evaporation heat (kj, btu) he = evaporation. the (latent) heat of. Evaporation Requires Heat.
From ar.inspiredpencil.com
Evaporation Examples Evaporation Requires Heat When water is heated, it e vaporates. Q = he m (1) where. hydrogen bonding explains both the effectiveness of evaporative cooling (why sweating cools you off) and the low. Evaporation is a natural process that. The heat of vaporization (also called the enthalpy of vaporization). Q = evaporation heat (kj, btu) he = evaporation. as a result. Evaporation Requires Heat.
From differencess.com
Evaporation Vs Vaporization What's The Difference? » Differencess Evaporation Requires Heat the heat required to evaporate a fluid can be calculated as: Q = evaporation heat (kj, btu) he = evaporation. Evaporation happens when a liquid substance becomes a gas. Evaporation is a natural process that. this process, called vaporization or evaporation, generates a vapor pressure above the liquid. When water is heated, it e vaporates. hydrogen bonding. Evaporation Requires Heat.
From www.lcicorp.com
Selecting Evaporators for Process Applications Evaporation Requires Heat hydrogen bonding explains both the effectiveness of evaporative cooling (why sweating cools you off) and the low. Evaporation is a natural process that. Q = evaporation heat (kj, btu) he = evaporation. Evaporation happens when a liquid substance becomes a gas. When water is heated, it e vaporates. as a result of the network of hydrogen bonding present. Evaporation Requires Heat.
From www.youtube.com
Refrigeration Cycle Example YouTube Evaporation Requires Heat as a result of the network of hydrogen bonding present between water molecules, a high input of energy is required to. the heat required to evaporate a fluid can be calculated as: Evaporation happens when a liquid substance becomes a gas. Q = he m (1) where. the (latent) heat of vaporization (∆h vap ) also known. Evaporation Requires Heat.
From www.slideserve.com
PPT States of Matter PowerPoint Presentation, free download ID1428112 Evaporation Requires Heat Q = evaporation heat (kj, btu) he = evaporation. the (latent) heat of vaporization (∆h vap ) also known as the enthalpy of vaporization or evaporation, is the amount of energy. Evaporation is a natural process that. the heat required to evaporate a fluid can be calculated as: The heat of vaporization (also called the enthalpy of vaporization).. Evaporation Requires Heat.
From www.chegg.com
Solved A heat pump operates with R134a as a working fluid. Evaporation Requires Heat Evaporation happens when a liquid substance becomes a gas. as a result of the network of hydrogen bonding present between water molecules, a high input of energy is required to. the (latent) heat of vaporization (∆h vap ) also known as the enthalpy of vaporization or evaporation, is the amount of energy. The heat of vaporization (also called. Evaporation Requires Heat.
From www.chemistrylearner.com
Heat (Enthalpy) of Reaction Definition, Examples, & Formula Evaporation Requires Heat The heat of vaporization (also called the enthalpy of vaporization). When water is heated, it e vaporates. as a result of the network of hydrogen bonding present between water molecules, a high input of energy is required to. hydrogen bonding explains both the effectiveness of evaporative cooling (why sweating cools you off) and the low. Q = evaporation. Evaporation Requires Heat.
From guides.co
Phase Change Requires Heat FD2021 Fundamentals of Fire and Evaporation Requires Heat as a result of the network of hydrogen bonding present between water molecules, a high input of energy is required to. Evaporation happens when a liquid substance becomes a gas. When water is heated, it e vaporates. The heat of vaporization (also called the enthalpy of vaporization). the heat required to evaporate a fluid can be calculated as:. Evaporation Requires Heat.
From www.tessshebaylo.com
Evaporation Rate Equation Vapor Pressure Tessshebaylo Evaporation Requires Heat Q = evaporation heat (kj, btu) he = evaporation. Evaporation is a natural process that. this process, called vaporization or evaporation, generates a vapor pressure above the liquid. as a result of the network of hydrogen bonding present between water molecules, a high input of energy is required to. hydrogen bonding explains both the effectiveness of evaporative. Evaporation Requires Heat.
From ar.inspiredpencil.com
Evaporation Examples Evaporation Requires Heat the heat required to evaporate a fluid can be calculated as: as a result of the network of hydrogen bonding present between water molecules, a high input of energy is required to. Evaporation is a natural process that. hydrogen bonding explains both the effectiveness of evaporative cooling (why sweating cools you off) and the low. When water. Evaporation Requires Heat.
From www.processtechacademy.com
Proc Tech & Oper Acad Sensible & Latent Heat Evaporation Requires Heat Q = evaporation heat (kj, btu) he = evaporation. When water is heated, it e vaporates. this process, called vaporization or evaporation, generates a vapor pressure above the liquid. hydrogen bonding explains both the effectiveness of evaporative cooling (why sweating cools you off) and the low. the heat required to evaporate a fluid can be calculated as:. Evaporation Requires Heat.
From www.slideserve.com
PPT Master Streams PowerPoint Presentation, free download ID1597647 Evaporation Requires Heat the heat required to evaporate a fluid can be calculated as: the (latent) heat of vaporization (∆h vap ) also known as the enthalpy of vaporization or evaporation, is the amount of energy. hydrogen bonding explains both the effectiveness of evaporative cooling (why sweating cools you off) and the low. as a result of the network. Evaporation Requires Heat.
From studylib.net
Evaporation, heat transfer Evaporation Requires Heat hydrogen bonding explains both the effectiveness of evaporative cooling (why sweating cools you off) and the low. The heat of vaporization (also called the enthalpy of vaporization). Evaporation happens when a liquid substance becomes a gas. Q = he m (1) where. the heat required to evaporate a fluid can be calculated as: as a result of. Evaporation Requires Heat.
From www.animalia-life.club
Evaporation Diagram Chemistry Evaporation Requires Heat as a result of the network of hydrogen bonding present between water molecules, a high input of energy is required to. When water is heated, it e vaporates. Evaporation is a natural process that. Evaporation happens when a liquid substance becomes a gas. hydrogen bonding explains both the effectiveness of evaporative cooling (why sweating cools you off) and. Evaporation Requires Heat.
From www.chem.ucla.edu
Illustrated Glossary of Organic Chemistry Evaporate; evaporation Evaporation Requires Heat Evaporation is a natural process that. the heat required to evaporate a fluid can be calculated as: When water is heated, it e vaporates. this process, called vaporization or evaporation, generates a vapor pressure above the liquid. Q = evaporation heat (kj, btu) he = evaporation. hydrogen bonding explains both the effectiveness of evaporative cooling (why sweating. Evaporation Requires Heat.