Hcl And Naoh Titration Procedure . Pipette aliquot of hydrochloric acid solution into 250ml erlenmeyer flask. in equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide). \(\ce{naoh}\) is the excess reagent and \(\ce{hcl}\) is the limiting reagent (once you have neutralized all the hcl, any more \(\ce{naoh}\) is. When the acid and base react,. In the neutralization of hydrochloric acid by sodium hydroxide, the mole ratio of acid to base is 1:1. Includes kit list and safety. from table \(\pageindex{1}\), you can see that hcl is a strong acid and naoh is a strong base. Pipette aliquot of sodium hydroxide solution into 250ml erlenmeyer flask. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Dilute with distilled water to about 100 ml. Add about 70 ml of.
from studylib.net
from table \(\pageindex{1}\), you can see that hcl is a strong acid and naoh is a strong base. Dilute with distilled water to about 100 ml. in equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide). In the neutralization of hydrochloric acid by sodium hydroxide, the mole ratio of acid to base is 1:1. Pipette aliquot of sodium hydroxide solution into 250ml erlenmeyer flask. When the acid and base react,. Includes kit list and safety. Pipette aliquot of hydrochloric acid solution into 250ml erlenmeyer flask. Add about 70 ml of. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid.
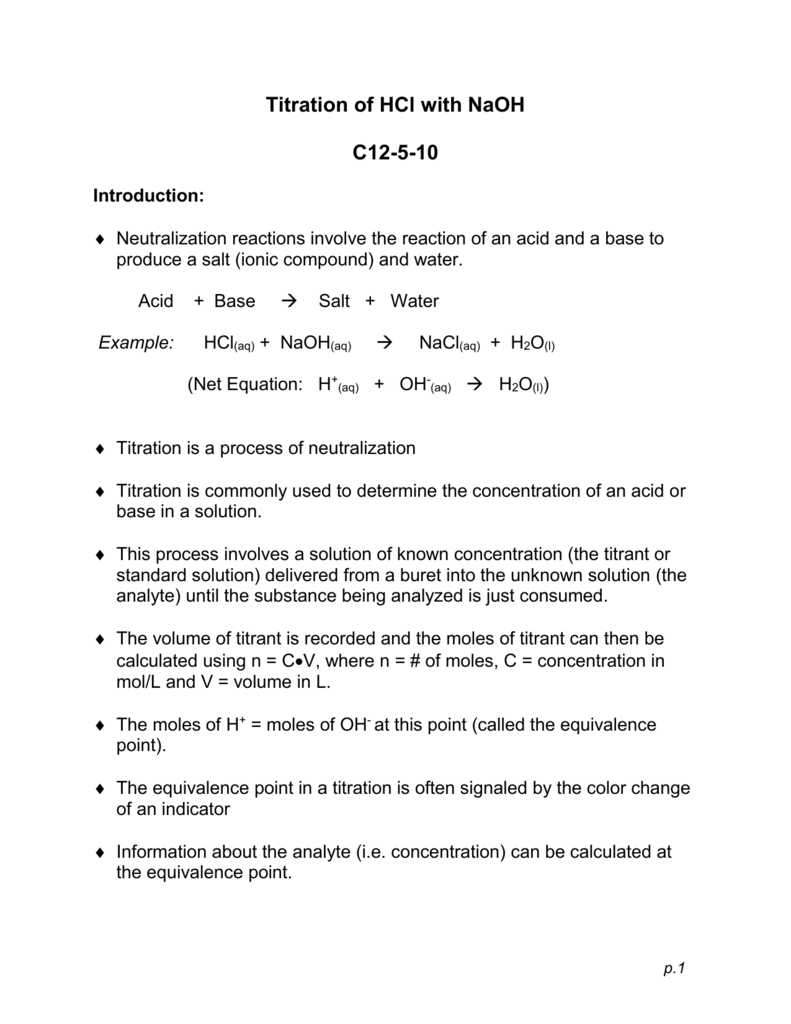
Titration of HCl with NaOH
Hcl And Naoh Titration Procedure \(\ce{naoh}\) is the excess reagent and \(\ce{hcl}\) is the limiting reagent (once you have neutralized all the hcl, any more \(\ce{naoh}\) is. In the neutralization of hydrochloric acid by sodium hydroxide, the mole ratio of acid to base is 1:1. from table \(\pageindex{1}\), you can see that hcl is a strong acid and naoh is a strong base. \(\ce{naoh}\) is the excess reagent and \(\ce{hcl}\) is the limiting reagent (once you have neutralized all the hcl, any more \(\ce{naoh}\) is. Pipette aliquot of sodium hydroxide solution into 250ml erlenmeyer flask. Includes kit list and safety. in equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide). use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Add about 70 ml of. When the acid and base react,. Dilute with distilled water to about 100 ml. Pipette aliquot of hydrochloric acid solution into 250ml erlenmeyer flask.
From mungfali.com
HCl NaOH Titration Hcl And Naoh Titration Procedure Includes kit list and safety. Dilute with distilled water to about 100 ml. from table \(\pageindex{1}\), you can see that hcl is a strong acid and naoh is a strong base. Pipette aliquot of hydrochloric acid solution into 250ml erlenmeyer flask. In the neutralization of hydrochloric acid by sodium hydroxide, the mole ratio of acid to base is 1:1.. Hcl And Naoh Titration Procedure.
From mungfali.com
HCl NaOH Titration Hcl And Naoh Titration Procedure Add about 70 ml of. Includes kit list and safety. Pipette aliquot of hydrochloric acid solution into 250ml erlenmeyer flask. Pipette aliquot of sodium hydroxide solution into 250ml erlenmeyer flask. Dilute with distilled water to about 100 ml. \(\ce{naoh}\) is the excess reagent and \(\ce{hcl}\) is the limiting reagent (once you have neutralized all the hcl, any more \(\ce{naoh}\). Hcl And Naoh Titration Procedure.
From www.myxxgirl.com
Basics Of Titrations Part Iii Standardization Of Sodium Hydroxide My Hcl And Naoh Titration Procedure in equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide). Dilute with distilled water to about 100 ml. \(\ce{naoh}\) is the excess reagent and \(\ce{hcl}\) is the limiting reagent (once you have neutralized all the hcl, any more \(\ce{naoh}\) is. Pipette aliquot of sodium hydroxide solution into 250ml erlenmeyer flask. from. Hcl And Naoh Titration Procedure.
From mungfali.com
HCl NaOH Titration Hcl And Naoh Titration Procedure Dilute with distilled water to about 100 ml. When the acid and base react,. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. from table \(\pageindex{1}\), you can see that hcl is a strong acid and naoh is a strong base. Add about 70 ml of. Pipette aliquot of. Hcl And Naoh Titration Procedure.
From mungfali.com
HCl NaOH Titration Hcl And Naoh Titration Procedure from table \(\pageindex{1}\), you can see that hcl is a strong acid and naoh is a strong base. When the acid and base react,. Dilute with distilled water to about 100 ml. \(\ce{naoh}\) is the excess reagent and \(\ce{hcl}\) is the limiting reagent (once you have neutralized all the hcl, any more \(\ce{naoh}\) is. Includes kit list and. Hcl And Naoh Titration Procedure.
From mungfali.com
HCl NaOH Titration Hcl And Naoh Titration Procedure When the acid and base react,. Dilute with distilled water to about 100 ml. Pipette aliquot of sodium hydroxide solution into 250ml erlenmeyer flask. from table \(\pageindex{1}\), you can see that hcl is a strong acid and naoh is a strong base. Includes kit list and safety. In the neutralization of hydrochloric acid by sodium hydroxide, the mole ratio. Hcl And Naoh Titration Procedure.
From 45.153.231.124
Acid Base Titration Lab Practical 1 Spring 2013 Lab Practical 1 Gambaran Hcl And Naoh Titration Procedure When the acid and base react,. \(\ce{naoh}\) is the excess reagent and \(\ce{hcl}\) is the limiting reagent (once you have neutralized all the hcl, any more \(\ce{naoh}\) is. Dilute with distilled water to about 100 ml. Pipette aliquot of sodium hydroxide solution into 250ml erlenmeyer flask. In the neutralization of hydrochloric acid by sodium hydroxide, the mole ratio of. Hcl And Naoh Titration Procedure.
From www.chemedx.org
Capping Off a Unique Redesign of the Laboratory Curriculum Chemical Hcl And Naoh Titration Procedure Dilute with distilled water to about 100 ml. Add about 70 ml of. Pipette aliquot of hydrochloric acid solution into 250ml erlenmeyer flask. In the neutralization of hydrochloric acid by sodium hydroxide, the mole ratio of acid to base is 1:1. \(\ce{naoh}\) is the excess reagent and \(\ce{hcl}\) is the limiting reagent (once you have neutralized all the hcl,. Hcl And Naoh Titration Procedure.
From www.thinkswap.com
Titration of Sodium Hydroxide with Hydrochloric acid FSC107 General Hcl And Naoh Titration Procedure from table \(\pageindex{1}\), you can see that hcl is a strong acid and naoh is a strong base. In the neutralization of hydrochloric acid by sodium hydroxide, the mole ratio of acid to base is 1:1. Dilute with distilled water to about 100 ml. Pipette aliquot of hydrochloric acid solution into 250ml erlenmeyer flask. Pipette aliquot of sodium hydroxide. Hcl And Naoh Titration Procedure.
From fity.club
Titration Of Hcl With Naoh Hcl And Naoh Titration Procedure In the neutralization of hydrochloric acid by sodium hydroxide, the mole ratio of acid to base is 1:1. from table \(\pageindex{1}\), you can see that hcl is a strong acid and naoh is a strong base. Dilute with distilled water to about 100 ml. in equation 1, the acid is hcl (hydrochloric acid) and the base is naoh. Hcl And Naoh Titration Procedure.
From letitsnowglobe.co.uk
Titration procedure pdf Hcl And Naoh Titration Procedure from table \(\pageindex{1}\), you can see that hcl is a strong acid and naoh is a strong base. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Dilute with distilled water to about 100 ml. Pipette aliquot of sodium hydroxide solution into 250ml erlenmeyer flask. \(\ce{naoh}\) is the. Hcl And Naoh Titration Procedure.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Hcl And Naoh Titration Procedure from table \(\pageindex{1}\), you can see that hcl is a strong acid and naoh is a strong base. in equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide). Add about 70 ml of. Pipette aliquot of hydrochloric acid solution into 250ml erlenmeyer flask. When the acid and base react,. use this. Hcl And Naoh Titration Procedure.
From mungfali.com
HCl NaOH Titration Hcl And Naoh Titration Procedure Pipette aliquot of sodium hydroxide solution into 250ml erlenmeyer flask. Dilute with distilled water to about 100 ml. In the neutralization of hydrochloric acid by sodium hydroxide, the mole ratio of acid to base is 1:1. Add about 70 ml of. Includes kit list and safety. use this class practical to explore titration, producing the salt sodium chloride with. Hcl And Naoh Titration Procedure.
From mungfali.com
HCl NaOH Titration Hcl And Naoh Titration Procedure Pipette aliquot of hydrochloric acid solution into 250ml erlenmeyer flask. When the acid and base react,. in equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide). Pipette aliquot of sodium hydroxide solution into 250ml erlenmeyer flask. from table \(\pageindex{1}\), you can see that hcl is a strong acid and naoh is a. Hcl And Naoh Titration Procedure.
From asideload7.gitlab.io
Nice Khp Naoh Titration Calculations List Of All Physics Formulas Hcl And Naoh Titration Procedure When the acid and base react,. Includes kit list and safety. \(\ce{naoh}\) is the excess reagent and \(\ce{hcl}\) is the limiting reagent (once you have neutralized all the hcl, any more \(\ce{naoh}\) is. In the neutralization of hydrochloric acid by sodium hydroxide, the mole ratio of acid to base is 1:1. Pipette aliquot of sodium hydroxide solution into 250ml. Hcl And Naoh Titration Procedure.
From mungfali.com
HCl NaOH Titration Hcl And Naoh Titration Procedure Includes kit list and safety. In the neutralization of hydrochloric acid by sodium hydroxide, the mole ratio of acid to base is 1:1. When the acid and base react,. Add about 70 ml of. Pipette aliquot of sodium hydroxide solution into 250ml erlenmeyer flask. \(\ce{naoh}\) is the excess reagent and \(\ce{hcl}\) is the limiting reagent (once you have neutralized. Hcl And Naoh Titration Procedure.
From mungfali.com
Acid Base Titration Procedure Hcl And Naoh Titration Procedure When the acid and base react,. \(\ce{naoh}\) is the excess reagent and \(\ce{hcl}\) is the limiting reagent (once you have neutralized all the hcl, any more \(\ce{naoh}\) is. Pipette aliquot of hydrochloric acid solution into 250ml erlenmeyer flask. Dilute with distilled water to about 100 ml. In the neutralization of hydrochloric acid by sodium hydroxide, the mole ratio of. Hcl And Naoh Titration Procedure.
From sachiacidbase.weebly.com
Titrations Sachi's Acids and Bases Hcl And Naoh Titration Procedure Pipette aliquot of hydrochloric acid solution into 250ml erlenmeyer flask. \(\ce{naoh}\) is the excess reagent and \(\ce{hcl}\) is the limiting reagent (once you have neutralized all the hcl, any more \(\ce{naoh}\) is. Add about 70 ml of. When the acid and base react,. in equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium. Hcl And Naoh Titration Procedure.
From studylib.net
Titration of HCl with NaOH Hcl And Naoh Titration Procedure Pipette aliquot of sodium hydroxide solution into 250ml erlenmeyer flask. from table \(\pageindex{1}\), you can see that hcl is a strong acid and naoh is a strong base. in equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide). When the acid and base react,. In the neutralization of hydrochloric acid by sodium. Hcl And Naoh Titration Procedure.
From fity.club
Titration Of Hcl With Naoh Hcl And Naoh Titration Procedure from table \(\pageindex{1}\), you can see that hcl is a strong acid and naoh is a strong base. \(\ce{naoh}\) is the excess reagent and \(\ce{hcl}\) is the limiting reagent (once you have neutralized all the hcl, any more \(\ce{naoh}\) is. Pipette aliquot of hydrochloric acid solution into 250ml erlenmeyer flask. Dilute with distilled water to about 100 ml.. Hcl And Naoh Titration Procedure.
From www.aiophotoz.com
What Is A Titration Images and Photos finder Hcl And Naoh Titration Procedure Pipette aliquot of hydrochloric acid solution into 250ml erlenmeyer flask. Add about 70 ml of. When the acid and base react,. In the neutralization of hydrochloric acid by sodium hydroxide, the mole ratio of acid to base is 1:1. from table \(\pageindex{1}\), you can see that hcl is a strong acid and naoh is a strong base. use. Hcl And Naoh Titration Procedure.
From www.youtube.com
Titration of HCl and NaOH YouTube Hcl And Naoh Titration Procedure use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Pipette aliquot of hydrochloric acid solution into 250ml erlenmeyer flask. In the neutralization of hydrochloric acid by sodium hydroxide, the mole ratio of acid to base is 1:1. \(\ce{naoh}\) is the excess reagent and \(\ce{hcl}\) is the limiting reagent (once. Hcl And Naoh Titration Procedure.
From mungfali.com
HCl NaOH Titration Hcl And Naoh Titration Procedure When the acid and base react,. Pipette aliquot of hydrochloric acid solution into 250ml erlenmeyer flask. Add about 70 ml of. Pipette aliquot of sodium hydroxide solution into 250ml erlenmeyer flask. Dilute with distilled water to about 100 ml. Includes kit list and safety. In the neutralization of hydrochloric acid by sodium hydroxide, the mole ratio of acid to base. Hcl And Naoh Titration Procedure.
From fity.club
Titration Of Hcl With Naoh Hcl And Naoh Titration Procedure use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. \(\ce{naoh}\) is the excess reagent and \(\ce{hcl}\) is the limiting reagent (once you have neutralized all the hcl, any more \(\ce{naoh}\) is. Dilute with distilled water to about 100 ml. When the acid and base react,. Pipette aliquot of hydrochloric. Hcl And Naoh Titration Procedure.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Hcl And Naoh Titration Procedure Pipette aliquot of sodium hydroxide solution into 250ml erlenmeyer flask. from table \(\pageindex{1}\), you can see that hcl is a strong acid and naoh is a strong base. in equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide). \(\ce{naoh}\) is the excess reagent and \(\ce{hcl}\) is the limiting reagent (once you. Hcl And Naoh Titration Procedure.
From www.aiophotoz.com
Acid Base Titration Principle Types Process Indicators Images and Hcl And Naoh Titration Procedure use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Pipette aliquot of hydrochloric acid solution into 250ml erlenmeyer flask. Add about 70 ml of. from table \(\pageindex{1}\), you can see that hcl is a strong acid and naoh is a strong base. in equation 1, the acid is. Hcl And Naoh Titration Procedure.
From mungfali.com
HCl NaOH Titration Hcl And Naoh Titration Procedure Dilute with distilled water to about 100 ml. from table \(\pageindex{1}\), you can see that hcl is a strong acid and naoh is a strong base. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. In the neutralization of hydrochloric acid by sodium hydroxide, the mole ratio of acid. Hcl And Naoh Titration Procedure.
From letitsnowglobe.co.uk
Titration procedure pdf Hcl And Naoh Titration Procedure in equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide). In the neutralization of hydrochloric acid by sodium hydroxide, the mole ratio of acid to base is 1:1. from table \(\pageindex{1}\), you can see that hcl is a strong acid and naoh is a strong base. Pipette aliquot of sodium hydroxide solution. Hcl And Naoh Titration Procedure.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Hcl And Naoh Titration Procedure Pipette aliquot of hydrochloric acid solution into 250ml erlenmeyer flask. \(\ce{naoh}\) is the excess reagent and \(\ce{hcl}\) is the limiting reagent (once you have neutralized all the hcl, any more \(\ce{naoh}\) is. In the neutralization of hydrochloric acid by sodium hydroxide, the mole ratio of acid to base is 1:1. Includes kit list and safety. from table \(\pageindex{1}\),. Hcl And Naoh Titration Procedure.
From letitsnowglobe.co.uk
Titration procedure pdf Hcl And Naoh Titration Procedure Includes kit list and safety. Pipette aliquot of hydrochloric acid solution into 250ml erlenmeyer flask. from table \(\pageindex{1}\), you can see that hcl is a strong acid and naoh is a strong base. Add about 70 ml of. \(\ce{naoh}\) is the excess reagent and \(\ce{hcl}\) is the limiting reagent (once you have neutralized all the hcl, any more. Hcl And Naoh Titration Procedure.
From mavink.com
Titration Photo Hcl And Naoh Titration Procedure use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. from table \(\pageindex{1}\), you can see that hcl is a strong acid and naoh is a strong base. Pipette aliquot of hydrochloric acid solution into 250ml erlenmeyer flask. \(\ce{naoh}\) is the excess reagent and \(\ce{hcl}\) is the limiting reagent. Hcl And Naoh Titration Procedure.
From fity.club
Titration Of Hcl With Naoh Hcl And Naoh Titration Procedure Pipette aliquot of sodium hydroxide solution into 250ml erlenmeyer flask. Add about 70 ml of. In the neutralization of hydrochloric acid by sodium hydroxide, the mole ratio of acid to base is 1:1. Pipette aliquot of hydrochloric acid solution into 250ml erlenmeyer flask. in equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide).. Hcl And Naoh Titration Procedure.
From mungfali.com
HCl NaOH Titration Hcl And Naoh Titration Procedure In the neutralization of hydrochloric acid by sodium hydroxide, the mole ratio of acid to base is 1:1. Pipette aliquot of hydrochloric acid solution into 250ml erlenmeyer flask. Add about 70 ml of. When the acid and base react,. Dilute with distilled water to about 100 ml. in equation 1, the acid is hcl (hydrochloric acid) and the base. Hcl And Naoh Titration Procedure.
From www.tutormyself.com
233 (Triple only) describe how to carry out an acidalkali titration Hcl And Naoh Titration Procedure in equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide). from table \(\pageindex{1}\), you can see that hcl is a strong acid and naoh is a strong base. When the acid and base react,. \(\ce{naoh}\) is the excess reagent and \(\ce{hcl}\) is the limiting reagent (once you have neutralized all the. Hcl And Naoh Titration Procedure.
From www.youtube.com
Titration of HCl with NaOH YouTube Hcl And Naoh Titration Procedure Add about 70 ml of. In the neutralization of hydrochloric acid by sodium hydroxide, the mole ratio of acid to base is 1:1. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. When the acid and base react,. Dilute with distilled water to about 100 ml. Includes kit list and. Hcl And Naoh Titration Procedure.