Magnesium Charge Density . magnesium the ionic radius for the +2 cation of magnesium is fairly small (0.65 å). for example, magnesium has a higher charge density compared to sodium, therefore its metallic lattice is stronger and it has a higher. Typical densities of various substances are at atmospheric pressure. density of magnesium is 1.738g/cm3. magnesium hydride is unfavorable for dehydrogenation processes. density (g cm −3) density is the mass of a substance that would fill 1 cm 3 at room temperature. charge densities of selected ions. Charge densities (c mm23) are calculated according to the formula. Understanding the bonding nature of mg and h is. As a consequence the charge density (z/r) is high,.
from slidetodoc.com
for example, magnesium has a higher charge density compared to sodium, therefore its metallic lattice is stronger and it has a higher. Understanding the bonding nature of mg and h is. magnesium the ionic radius for the +2 cation of magnesium is fairly small (0.65 å). magnesium hydride is unfavorable for dehydrogenation processes. Charge densities (c mm23) are calculated according to the formula. Typical densities of various substances are at atmospheric pressure. density of magnesium is 1.738g/cm3. charge densities of selected ions. density (g cm −3) density is the mass of a substance that would fill 1 cm 3 at room temperature. As a consequence the charge density (z/r) is high,.
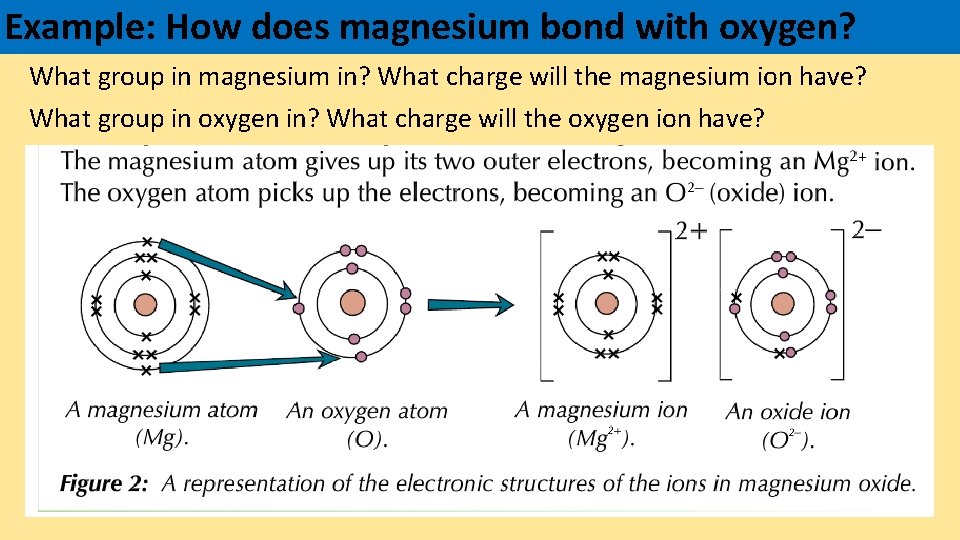
Metal ions Nonmetal ions Positive ion Gain electrons
Magnesium Charge Density charge densities of selected ions. Understanding the bonding nature of mg and h is. magnesium hydride is unfavorable for dehydrogenation processes. magnesium the ionic radius for the +2 cation of magnesium is fairly small (0.65 å). Typical densities of various substances are at atmospheric pressure. Charge densities (c mm23) are calculated according to the formula. density of magnesium is 1.738g/cm3. density (g cm −3) density is the mass of a substance that would fill 1 cm 3 at room temperature. for example, magnesium has a higher charge density compared to sodium, therefore its metallic lattice is stronger and it has a higher. charge densities of selected ions. As a consequence the charge density (z/r) is high,.
From www.dreamstime.com
Magnesium Atom, with Mass and Energy Levels. Stock Vector Magnesium Charge Density density (g cm −3) density is the mass of a substance that would fill 1 cm 3 at room temperature. As a consequence the charge density (z/r) is high,. Understanding the bonding nature of mg and h is. magnesium the ionic radius for the +2 cation of magnesium is fairly small (0.65 å). density of magnesium is. Magnesium Charge Density.
From www.researchgate.net
Energy density range of the SLMed magnesium and its alloys Magnesium Charge Density charge densities of selected ions. Charge densities (c mm23) are calculated according to the formula. Understanding the bonding nature of mg and h is. density (g cm −3) density is the mass of a substance that would fill 1 cm 3 at room temperature. magnesium hydride is unfavorable for dehydrogenation processes. Typical densities of various substances are. Magnesium Charge Density.
From www.smart-elements.com
Magnesium precision density cube 10mm 1.74g Magnesium Charge Density Typical densities of various substances are at atmospheric pressure. magnesium the ionic radius for the +2 cation of magnesium is fairly small (0.65 å). As a consequence the charge density (z/r) is high,. Understanding the bonding nature of mg and h is. for example, magnesium has a higher charge density compared to sodium, therefore its metallic lattice is. Magnesium Charge Density.
From www.nuclear-power.com
Magnesium Atomic Number Atomic Mass Density of Magnesium Magnesium Charge Density magnesium the ionic radius for the +2 cation of magnesium is fairly small (0.65 å). Charge densities (c mm23) are calculated according to the formula. Typical densities of various substances are at atmospheric pressure. density (g cm −3) density is the mass of a substance that would fill 1 cm 3 at room temperature. As a consequence the. Magnesium Charge Density.
From www.researchgate.net
a) 3D isosurface representation of charge density (0.02 e Å À1 ), b Magnesium Charge Density charge densities of selected ions. Typical densities of various substances are at atmospheric pressure. Understanding the bonding nature of mg and h is. density of magnesium is 1.738g/cm3. magnesium the ionic radius for the +2 cation of magnesium is fairly small (0.65 å). As a consequence the charge density (z/r) is high,. for example, magnesium has. Magnesium Charge Density.
From www.researchgate.net
(a) Tunability of charge carrier concentration of ZnMgON by the Magnesium Charge Density magnesium the ionic radius for the +2 cation of magnesium is fairly small (0.65 å). Understanding the bonding nature of mg and h is. density (g cm −3) density is the mass of a substance that would fill 1 cm 3 at room temperature. Typical densities of various substances are at atmospheric pressure. charge densities of selected. Magnesium Charge Density.
From slideplayer.com
Investigation of formability of super plastic magnesium alloy ppt Magnesium Charge Density Understanding the bonding nature of mg and h is. magnesium hydride is unfavorable for dehydrogenation processes. charge densities of selected ions. for example, magnesium has a higher charge density compared to sodium, therefore its metallic lattice is stronger and it has a higher. magnesium the ionic radius for the +2 cation of magnesium is fairly small. Magnesium Charge Density.
From www.gkseries.com
The chemistry of lithium is very similar to that of magnesium even Magnesium Charge Density charge densities of selected ions. As a consequence the charge density (z/r) is high,. for example, magnesium has a higher charge density compared to sodium, therefore its metallic lattice is stronger and it has a higher. magnesium hydride is unfavorable for dehydrogenation processes. magnesium the ionic radius for the +2 cation of magnesium is fairly small. Magnesium Charge Density.
From www.researchgate.net
Temperature dependence of heat capacity of magnesium in solid and Magnesium Charge Density As a consequence the charge density (z/r) is high,. density of magnesium is 1.738g/cm3. density (g cm −3) density is the mass of a substance that would fill 1 cm 3 at room temperature. Understanding the bonding nature of mg and h is. Typical densities of various substances are at atmospheric pressure. charge densities of selected ions.. Magnesium Charge Density.
From www.dreamstime.com
Fully Labelled Illustration of Magnesium Reaction with Steam Stock Magnesium Charge Density magnesium hydride is unfavorable for dehydrogenation processes. Charge densities (c mm23) are calculated according to the formula. density of magnesium is 1.738g/cm3. magnesium the ionic radius for the +2 cation of magnesium is fairly small (0.65 å). Typical densities of various substances are at atmospheric pressure. Understanding the bonding nature of mg and h is. density. Magnesium Charge Density.
From www.smart-elements.com
BIG high precision Magnesium densitystandard cube 10cm3 17.4g Magnesium Charge Density Typical densities of various substances are at atmospheric pressure. Charge densities (c mm23) are calculated according to the formula. density of magnesium is 1.738g/cm3. charge densities of selected ions. Understanding the bonding nature of mg and h is. for example, magnesium has a higher charge density compared to sodium, therefore its metallic lattice is stronger and it. Magnesium Charge Density.
From www.elevise.co.uk
C2 A) Ionic Bonds AQA Combined Science Trilogy Elevise Magnesium Charge Density Charge densities (c mm23) are calculated according to the formula. As a consequence the charge density (z/r) is high,. density of magnesium is 1.738g/cm3. density (g cm −3) density is the mass of a substance that would fill 1 cm 3 at room temperature. Typical densities of various substances are at atmospheric pressure. magnesium hydride is unfavorable. Magnesium Charge Density.
From www.researchgate.net
Phase diagram of MgZn system [1] Download Scientific Diagram Magnesium Charge Density for example, magnesium has a higher charge density compared to sodium, therefore its metallic lattice is stronger and it has a higher. density of magnesium is 1.738g/cm3. density (g cm −3) density is the mass of a substance that would fill 1 cm 3 at room temperature. magnesium the ionic radius for the +2 cation of. Magnesium Charge Density.
From www.toppr.com
How is magnesium chloride formed by the transfer of electrons? Why does Magnesium Charge Density density (g cm −3) density is the mass of a substance that would fill 1 cm 3 at room temperature. Typical densities of various substances are at atmospheric pressure. magnesium the ionic radius for the +2 cation of magnesium is fairly small (0.65 å). magnesium hydride is unfavorable for dehydrogenation processes. Understanding the bonding nature of mg. Magnesium Charge Density.
From www.britannica.com
magnesium Description, Properties, & Compounds Britannica Magnesium Charge Density density of magnesium is 1.738g/cm3. Charge densities (c mm23) are calculated according to the formula. Understanding the bonding nature of mg and h is. As a consequence the charge density (z/r) is high,. for example, magnesium has a higher charge density compared to sodium, therefore its metallic lattice is stronger and it has a higher. Typical densities of. Magnesium Charge Density.
From slidetodoc.com
Metal ions Nonmetal ions Positive ion Gain electrons Magnesium Charge Density density of magnesium is 1.738g/cm3. for example, magnesium has a higher charge density compared to sodium, therefore its metallic lattice is stronger and it has a higher. Understanding the bonding nature of mg and h is. Charge densities (c mm23) are calculated according to the formula. magnesium the ionic radius for the +2 cation of magnesium is. Magnesium Charge Density.
From www.researchgate.net
(PDF) Densities and molar volumes of solid lithiummagnesium (LiMg) alloys Magnesium Charge Density magnesium the ionic radius for the +2 cation of magnesium is fairly small (0.65 å). charge densities of selected ions. density of magnesium is 1.738g/cm3. As a consequence the charge density (z/r) is high,. Typical densities of various substances are at atmospheric pressure. Charge densities (c mm23) are calculated according to the formula. density (g cm. Magnesium Charge Density.
From www.openaccessgovernment.org
Insights into magnesium batteries using calorimetry Magnesium Charge Density magnesium hydride is unfavorable for dehydrogenation processes. magnesium the ionic radius for the +2 cation of magnesium is fairly small (0.65 å). for example, magnesium has a higher charge density compared to sodium, therefore its metallic lattice is stronger and it has a higher. Charge densities (c mm23) are calculated according to the formula. charge densities. Magnesium Charge Density.
From www.researchgate.net
(PDF) Thermophysical Properties of Magnesium in Solid and Liquid States Magnesium Charge Density density of magnesium is 1.738g/cm3. density (g cm −3) density is the mass of a substance that would fill 1 cm 3 at room temperature. Charge densities (c mm23) are calculated according to the formula. Typical densities of various substances are at atmospheric pressure. As a consequence the charge density (z/r) is high,. for example, magnesium has. Magnesium Charge Density.
From www.researchgate.net
Charge density distribution map of SnS in the (a) (100) and (b) (010 Magnesium Charge Density for example, magnesium has a higher charge density compared to sodium, therefore its metallic lattice is stronger and it has a higher. Understanding the bonding nature of mg and h is. magnesium hydride is unfavorable for dehydrogenation processes. density of magnesium is 1.738g/cm3. charge densities of selected ions. Charge densities (c mm23) are calculated according to. Magnesium Charge Density.
From www.researchgate.net
(PDF) Charge Density Analysis in Magnesium Hydride Magnesium Charge Density magnesium hydride is unfavorable for dehydrogenation processes. As a consequence the charge density (z/r) is high,. Typical densities of various substances are at atmospheric pressure. density (g cm −3) density is the mass of a substance that would fill 1 cm 3 at room temperature. magnesium the ionic radius for the +2 cation of magnesium is fairly. Magnesium Charge Density.
From www.researchgate.net
Parity plot for the charge density using magnesium chloride with Magnesium Charge Density charge densities of selected ions. Understanding the bonding nature of mg and h is. magnesium the ionic radius for the +2 cation of magnesium is fairly small (0.65 å). Charge densities (c mm23) are calculated according to the formula. As a consequence the charge density (z/r) is high,. magnesium hydride is unfavorable for dehydrogenation processes. Typical densities. Magnesium Charge Density.
From www.researchgate.net
(PDF) Densities of solid aluminummagnesium (AIMg) alloys Magnesium Charge Density As a consequence the charge density (z/r) is high,. Understanding the bonding nature of mg and h is. for example, magnesium has a higher charge density compared to sodium, therefore its metallic lattice is stronger and it has a higher. Typical densities of various substances are at atmospheric pressure. charge densities of selected ions. density (g cm. Magnesium Charge Density.
From www.researchgate.net
7 The charge density for the magnesium layer, in MgB 2 . The charge Magnesium Charge Density density of magnesium is 1.738g/cm3. magnesium the ionic radius for the +2 cation of magnesium is fairly small (0.65 å). charge densities of selected ions. As a consequence the charge density (z/r) is high,. density (g cm −3) density is the mass of a substance that would fill 1 cm 3 at room temperature. Understanding the. Magnesium Charge Density.
From journals.sagepub.com
Magnesiumion batteries for electric vehicles Current trends and Magnesium Charge Density Charge densities (c mm23) are calculated according to the formula. density (g cm −3) density is the mass of a substance that would fill 1 cm 3 at room temperature. charge densities of selected ions. density of magnesium is 1.738g/cm3. magnesium hydride is unfavorable for dehydrogenation processes. Understanding the bonding nature of mg and h is.. Magnesium Charge Density.
From valenceelectrons.com
Magnesium Electron Configuration Aufbau & Bohr Model Magnesium Charge Density Charge densities (c mm23) are calculated according to the formula. magnesium hydride is unfavorable for dehydrogenation processes. charge densities of selected ions. for example, magnesium has a higher charge density compared to sodium, therefore its metallic lattice is stronger and it has a higher. Typical densities of various substances are at atmospheric pressure. density (g cm. Magnesium Charge Density.
From solvedlib.com
Calculate the density of magnesium metal if 860 cm3 b… SolvedLib Magnesium Charge Density density of magnesium is 1.738g/cm3. Charge densities (c mm23) are calculated according to the formula. magnesium the ionic radius for the +2 cation of magnesium is fairly small (0.65 å). for example, magnesium has a higher charge density compared to sodium, therefore its metallic lattice is stronger and it has a higher. As a consequence the charge. Magnesium Charge Density.
From www.researchgate.net
Charge density distribution near the Fermi level (a,b) and density of Magnesium Charge Density magnesium hydride is unfavorable for dehydrogenation processes. Typical densities of various substances are at atmospheric pressure. As a consequence the charge density (z/r) is high,. Understanding the bonding nature of mg and h is. density of magnesium is 1.738g/cm3. charge densities of selected ions. density (g cm −3) density is the mass of a substance that. Magnesium Charge Density.
From www.researchgate.net
Charge density of I4/mmm type MgH4 (a) interaction between magnesium Magnesium Charge Density for example, magnesium has a higher charge density compared to sodium, therefore its metallic lattice is stronger and it has a higher. charge densities of selected ions. magnesium hydride is unfavorable for dehydrogenation processes. As a consequence the charge density (z/r) is high,. Charge densities (c mm23) are calculated according to the formula. Typical densities of various. Magnesium Charge Density.
From www.youtube.com
How to Find the Ionic Charge for Magnesium (Mg) YouTube Magnesium Charge Density density (g cm −3) density is the mass of a substance that would fill 1 cm 3 at room temperature. As a consequence the charge density (z/r) is high,. Typical densities of various substances are at atmospheric pressure. Understanding the bonding nature of mg and h is. magnesium the ionic radius for the +2 cation of magnesium is. Magnesium Charge Density.
From www.researchgate.net
(a) Band structure, (b) Density of states, (c) Charge density map and Magnesium Charge Density charge densities of selected ions. magnesium the ionic radius for the +2 cation of magnesium is fairly small (0.65 å). As a consequence the charge density (z/r) is high,. Typical densities of various substances are at atmospheric pressure. Understanding the bonding nature of mg and h is. for example, magnesium has a higher charge density compared to. Magnesium Charge Density.
From www.researchgate.net
Charge density distribution for (a) pristine and (b) Naintercalated Magnesium Charge Density for example, magnesium has a higher charge density compared to sodium, therefore its metallic lattice is stronger and it has a higher. Understanding the bonding nature of mg and h is. density (g cm −3) density is the mass of a substance that would fill 1 cm 3 at room temperature. density of magnesium is 1.738g/cm3. Typical. Magnesium Charge Density.
From dxohjpizz.blob.core.windows.net
Magnesium Ion Have A Charge at Robert Ezell blog Magnesium Charge Density Charge densities (c mm23) are calculated according to the formula. Typical densities of various substances are at atmospheric pressure. density (g cm −3) density is the mass of a substance that would fill 1 cm 3 at room temperature. density of magnesium is 1.738g/cm3. for example, magnesium has a higher charge density compared to sodium, therefore its. Magnesium Charge Density.
From www.slideshare.net
Bonding Basics Magnesium Charge Density Typical densities of various substances are at atmospheric pressure. Understanding the bonding nature of mg and h is. magnesium the ionic radius for the +2 cation of magnesium is fairly small (0.65 å). charge densities of selected ions. magnesium hydride is unfavorable for dehydrogenation processes. density of magnesium is 1.738g/cm3. Charge densities (c mm23) are calculated. Magnesium Charge Density.
From chemistry.stackexchange.com
experimental chemistry Calculating the density of a magnesium Magnesium Charge Density charge densities of selected ions. As a consequence the charge density (z/r) is high,. density (g cm −3) density is the mass of a substance that would fill 1 cm 3 at room temperature. Understanding the bonding nature of mg and h is. magnesium the ionic radius for the +2 cation of magnesium is fairly small (0.65. Magnesium Charge Density.