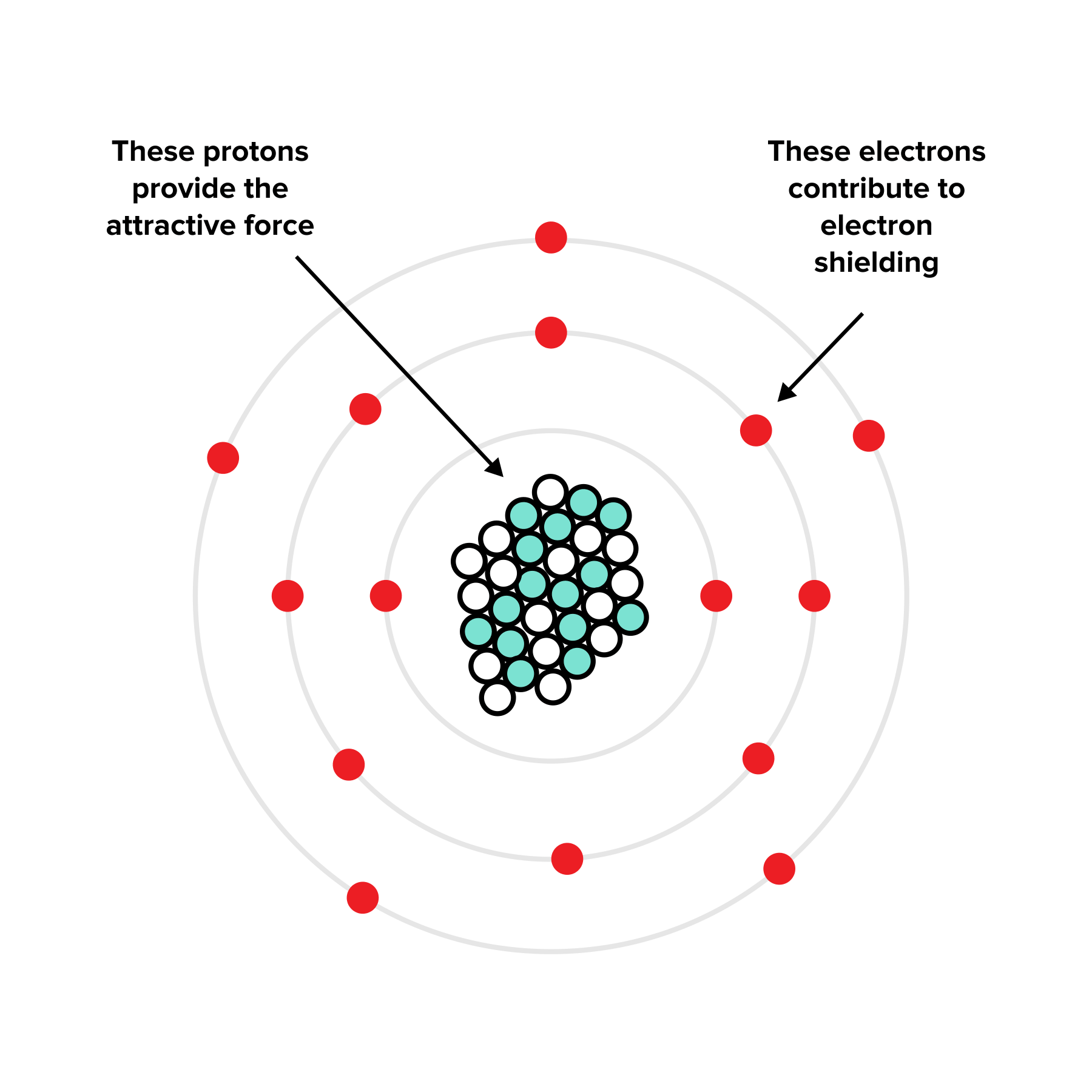
Which Type Of Electrons Are Best At Shielding A 3P Electron . the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. core electrons are adept at shielding, while electrons in the same valence shell do not block the nuclear attraction experienced by each other as efficiently. the electron being removed from an \(\ce{al}\) atom is a \(3p\) electron, which is shielded by the two \(3s\) electrons, as well as all the inner. shielding is caused by the combination of partial neutralization of nuclear charge by core electrons, and by electron. Therefore, it is easier to remove the outermost electron from. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use.
from www.shemmassianconsulting.com
shielding is caused by the combination of partial neutralization of nuclear charge by core electrons, and by electron. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. core electrons are adept at shielding, while electrons in the same valence shell do not block the nuclear attraction experienced by each other as efficiently. Therefore, it is easier to remove the outermost electron from. the electron being removed from an \(\ce{al}\) atom is a \(3p\) electron, which is shielded by the two \(3s\) electrons, as well as all the inner. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use.
Atoms and Periodic Trends for the MCAT Everything You Need to Know
Which Type Of Electrons Are Best At Shielding A 3P Electron the electron being removed from an \(\ce{al}\) atom is a \(3p\) electron, which is shielded by the two \(3s\) electrons, as well as all the inner. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. Therefore, it is easier to remove the outermost electron from. the electron being removed from an \(\ce{al}\) atom is a \(3p\) electron, which is shielded by the two \(3s\) electrons, as well as all the inner. shielding is caused by the combination of partial neutralization of nuclear charge by core electrons, and by electron. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. core electrons are adept at shielding, while electrons in the same valence shell do not block the nuclear attraction experienced by each other as efficiently.
From slideplayer.com
Periodic Trends Chemistry I. ppt download Which Type Of Electrons Are Best At Shielding A 3P Electron the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. core electrons are adept at shielding, while electrons in the same valence shell do not block the nuclear attraction experienced by each other as efficiently. the electron being removed from an \(\ce{al}\) atom is. Which Type Of Electrons Are Best At Shielding A 3P Electron.
From www.slideserve.com
PPT Lesson objectives • Define first ionisation energy and successive Which Type Of Electrons Are Best At Shielding A 3P Electron core electrons are adept at shielding, while electrons in the same valence shell do not block the nuclear attraction experienced by each other as efficiently. Therefore, it is easier to remove the outermost electron from. shielding is caused by the combination of partial neutralization of nuclear charge by core electrons, and by electron. the concept of electron. Which Type Of Electrons Are Best At Shielding A 3P Electron.
From fineartamerica.com
3p Electron Orbitals Photograph by Dr Mark J. Winter Which Type Of Electrons Are Best At Shielding A 3P Electron the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. Therefore, it is easier to remove the outermost electron from. the. Which Type Of Electrons Are Best At Shielding A 3P Electron.
From www.showme.com
Electron shielding Science, Chemistry, Electrons, electron shielding Which Type Of Electrons Are Best At Shielding A 3P Electron the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. shielding is caused by the combination of partial neutralization of nuclear charge by core electrons, and by electron. Therefore, it is easier to remove the outermost electron from. core electrons are adept at shielding,. Which Type Of Electrons Are Best At Shielding A 3P Electron.
From www.britannica.com
Electronic configuration Definition, Orbitals, & Facts Britannica Which Type Of Electrons Are Best At Shielding A 3P Electron the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. core electrons are adept at shielding, while electrons in the same valence shell do not block the nuclear attraction experienced by each other as efficiently. the concept of electron shielding, in which intervening electrons. Which Type Of Electrons Are Best At Shielding A 3P Electron.
From slideplayer.com
Chapter 7 Periodic Properties of the Elements ppt download Which Type Of Electrons Are Best At Shielding A 3P Electron Therefore, it is easier to remove the outermost electron from. core electrons are adept at shielding, while electrons in the same valence shell do not block the nuclear attraction experienced by each other as efficiently. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use.. Which Type Of Electrons Are Best At Shielding A 3P Electron.
From www.slideserve.com
PPT Periodic Table PowerPoint Presentation, free download ID2617702 Which Type Of Electrons Are Best At Shielding A 3P Electron the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. core electrons are adept at shielding, while electrons in the same valence shell do not block the nuclear attraction experienced by each other as efficiently. shielding is caused by the combination of partial neutralization. Which Type Of Electrons Are Best At Shielding A 3P Electron.
From byjus.com
What is shielding and deshielding in NMR? Give an example? Which Type Of Electrons Are Best At Shielding A 3P Electron the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. the electron being removed from an \(\ce{al}\) atom is a \(3p\). Which Type Of Electrons Are Best At Shielding A 3P Electron.
From slideplayer.com
Coulomb’s Law PERIODIC TRENDS Nuclear Charge Electron Shielding ppt Which Type Of Electrons Are Best At Shielding A 3P Electron the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. shielding is caused by the combination of partial neutralization of nuclear charge by core electrons, and by electron. the electron being removed from an \(\ce{al}\) atom is a \(3p\) electron, which is shielded by. Which Type Of Electrons Are Best At Shielding A 3P Electron.
From www.slideserve.com
PPT Periodic Trends PowerPoint Presentation, free download ID2836425 Which Type Of Electrons Are Best At Shielding A 3P Electron shielding is caused by the combination of partial neutralization of nuclear charge by core electrons, and by electron. the electron being removed from an \(\ce{al}\) atom is a \(3p\) electron, which is shielded by the two \(3s\) electrons, as well as all the inner. the concept of electron shielding, in which intervening electrons act to reduce the. Which Type Of Electrons Are Best At Shielding A 3P Electron.
From www2.chemistry.msu.edu
Electron Configurations & The Periodic Table Which Type Of Electrons Are Best At Shielding A 3P Electron the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. Therefore, it is easier to remove the outermost electron from. shielding is caused by the combination of partial neutralization of nuclear charge by core electrons, and by electron. core electrons are adept at shielding,. Which Type Of Electrons Are Best At Shielding A 3P Electron.
From slideplayer.com
Elemental Properties and Patterns ppt download Which Type Of Electrons Are Best At Shielding A 3P Electron shielding is caused by the combination of partial neutralization of nuclear charge by core electrons, and by electron. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. Therefore, it is easier to remove the outermost electron from. the electron being removed from an. Which Type Of Electrons Are Best At Shielding A 3P Electron.
From icdsc.org
How many electrons are in each shell including 3p orbitals Which Type Of Electrons Are Best At Shielding A 3P Electron core electrons are adept at shielding, while electrons in the same valence shell do not block the nuclear attraction experienced by each other as efficiently. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. shielding is caused by the combination of partial neutralization. Which Type Of Electrons Are Best At Shielding A 3P Electron.
From www.youtube.com
Shielding effect of Electroninner Electrons on outer Electronin the Which Type Of Electrons Are Best At Shielding A 3P Electron Therefore, it is easier to remove the outermost electron from. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. shielding is caused by the combination of partial neutralization of nuclear charge by core electrons, and by electron. the electron being removed from an. Which Type Of Electrons Are Best At Shielding A 3P Electron.
From www.slideserve.com
PPT The Periodic Table and Physical Properties PowerPoint Which Type Of Electrons Are Best At Shielding A 3P Electron the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. core electrons are adept at shielding, while electrons in the same valence shell do not block the nuclear attraction experienced by each other as efficiently. the concept of electron shielding, in which intervening electrons. Which Type Of Electrons Are Best At Shielding A 3P Electron.
From icdsc.org
How many electrons are in each shell including 3p orbitals Which Type Of Electrons Are Best At Shielding A 3P Electron the electron being removed from an \(\ce{al}\) atom is a \(3p\) electron, which is shielded by the two \(3s\) electrons, as well as all the inner. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. Therefore, it is easier to remove the outermost electron. Which Type Of Electrons Are Best At Shielding A 3P Electron.
From www.expii.com
Electrons — Structure & Properties Expii Which Type Of Electrons Are Best At Shielding A 3P Electron core electrons are adept at shielding, while electrons in the same valence shell do not block the nuclear attraction experienced by each other as efficiently. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. Therefore, it is easier to remove the outermost electron from.. Which Type Of Electrons Are Best At Shielding A 3P Electron.
From www.sciencephoto.com
3p electron orbitals Stock Image A152/0157 Science Photo Library Which Type Of Electrons Are Best At Shielding A 3P Electron the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. core electrons are adept at shielding, while electrons in the same valence shell do not block the nuclear attraction experienced by each other as efficiently. the concept of electron shielding, in which intervening electrons. Which Type Of Electrons Are Best At Shielding A 3P Electron.
From www.chem.fsu.edu
Electron Configurations Which Type Of Electrons Are Best At Shielding A 3P Electron Therefore, it is easier to remove the outermost electron from. shielding is caused by the combination of partial neutralization of nuclear charge by core electrons, and by electron. core electrons are adept at shielding, while electrons in the same valence shell do not block the nuclear attraction experienced by each other as efficiently. the concept of electron. Which Type Of Electrons Are Best At Shielding A 3P Electron.
From chemistry.stackexchange.com
chemistry My book's claim about the shielding effect of s,p Which Type Of Electrons Are Best At Shielding A 3P Electron Therefore, it is easier to remove the outermost electron from. the electron being removed from an \(\ce{al}\) atom is a \(3p\) electron, which is shielded by the two \(3s\) electrons, as well as all the inner. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the. Which Type Of Electrons Are Best At Shielding A 3P Electron.
From www.chemistrystudent.com
Electron Orbitals (ALevel) ChemistryStudent Which Type Of Electrons Are Best At Shielding A 3P Electron the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. shielding is caused by the combination of partial neutralization of nuclear charge by core electrons, and by electron. Therefore, it is easier to remove the outermost electron from. the electron being removed from an. Which Type Of Electrons Are Best At Shielding A 3P Electron.
From www.shemmassianconsulting.com
Atoms and Periodic Trends for the MCAT Everything You Need to Know Which Type Of Electrons Are Best At Shielding A 3P Electron the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. shielding is caused by the combination of partial neutralization of nuclear. Which Type Of Electrons Are Best At Shielding A 3P Electron.
From surfguppy.com
What is Electronegativity? Which Type Of Electrons Are Best At Shielding A 3P Electron the electron being removed from an \(\ce{al}\) atom is a \(3p\) electron, which is shielded by the two \(3s\) electrons, as well as all the inner. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. Therefore, it is easier to remove the outermost electron. Which Type Of Electrons Are Best At Shielding A 3P Electron.
From general.chemistrysteps.com
Ionization energy Chemistry Steps Which Type Of Electrons Are Best At Shielding A 3P Electron the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. the electron being removed from an \(\ce{al}\) atom is a \(3p\) electron, which is shielded by the two \(3s\) electrons, as well as all the inner. Therefore, it is easier to remove the outermost electron. Which Type Of Electrons Are Best At Shielding A 3P Electron.
From www.youtube.com
Electron shielding YouTube Which Type Of Electrons Are Best At Shielding A 3P Electron the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. Therefore, it is easier to remove the outermost electron from. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. shielding. Which Type Of Electrons Are Best At Shielding A 3P Electron.
From exoeftfzx.blob.core.windows.net
What Is Shielding Chem at Victor Riggs blog Which Type Of Electrons Are Best At Shielding A 3P Electron shielding is caused by the combination of partial neutralization of nuclear charge by core electrons, and by electron. core electrons are adept at shielding, while electrons in the same valence shell do not block the nuclear attraction experienced by each other as efficiently. the electron being removed from an \(\ce{al}\) atom is a \(3p\) electron, which is. Which Type Of Electrons Are Best At Shielding A 3P Electron.
From www.breakingatom.com
Shielding Which Type Of Electrons Are Best At Shielding A 3P Electron the electron being removed from an \(\ce{al}\) atom is a \(3p\) electron, which is shielded by the two \(3s\) electrons, as well as all the inner. core electrons are adept at shielding, while electrons in the same valence shell do not block the nuclear attraction experienced by each other as efficiently. the concept of electron shielding, in. Which Type Of Electrons Are Best At Shielding A 3P Electron.
From chemistnotes.com
Shielding Effect or Screening Effect Definition, Factors Affecting Which Type Of Electrons Are Best At Shielding A 3P Electron core electrons are adept at shielding, while electrons in the same valence shell do not block the nuclear attraction experienced by each other as efficiently. the electron being removed from an \(\ce{al}\) atom is a \(3p\) electron, which is shielded by the two \(3s\) electrons, as well as all the inner. shielding is caused by the combination. Which Type Of Electrons Are Best At Shielding A 3P Electron.
From newtondesk.com
Periodic Elements Electron Shells, SubShells, and Orbitals Chemistry Which Type Of Electrons Are Best At Shielding A 3P Electron the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. the electron being removed from an \(\ce{al}\) atom is a \(3p\) electron, which is shielded by the two \(3s\) electrons, as well as all the inner. the concept of electron shielding, in which intervening. Which Type Of Electrons Are Best At Shielding A 3P Electron.
From www.youtube.com
Chapter 03 05 Inner Electrons and Shielding YouTube Which Type Of Electrons Are Best At Shielding A 3P Electron the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. shielding is caused by the combination of partial neutralization of nuclear charge by core electrons, and by electron. core electrons are adept at shielding, while electrons in the same valence shell do not block. Which Type Of Electrons Are Best At Shielding A 3P Electron.
From sciencenotes.org
List of Electron Configurations of Elements Which Type Of Electrons Are Best At Shielding A 3P Electron the electron being removed from an \(\ce{al}\) atom is a \(3p\) electron, which is shielded by the two \(3s\) electrons, as well as all the inner. Therefore, it is easier to remove the outermost electron from. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the. Which Type Of Electrons Are Best At Shielding A 3P Electron.
From wisc.pb.unizin.org
M7Q8 Core and Valence Electrons, Shielding, Zeff Chem 103/104 Which Type Of Electrons Are Best At Shielding A 3P Electron shielding is caused by the combination of partial neutralization of nuclear charge by core electrons, and by electron. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. the electron being removed from an \(\ce{al}\) atom is a \(3p\) electron, which is shielded by. Which Type Of Electrons Are Best At Shielding A 3P Electron.
From www.youtube.com
S3.1.3 Electron shielding and effective nuclear charge YouTube Which Type Of Electrons Are Best At Shielding A 3P Electron Therefore, it is easier to remove the outermost electron from. shielding is caused by the combination of partial neutralization of nuclear charge by core electrons, and by electron. the electron being removed from an \(\ce{al}\) atom is a \(3p\) electron, which is shielded by the two \(3s\) electrons, as well as all the inner. the concept of. Which Type Of Electrons Are Best At Shielding A 3P Electron.
From www.slideserve.com
PPT Periodic Table Chapter PowerPoint Presentation ID3646738 Which Type Of Electrons Are Best At Shielding A 3P Electron the electron being removed from an \(\ce{al}\) atom is a \(3p\) electron, which is shielded by the two \(3s\) electrons, as well as all the inner. Therefore, it is easier to remove the outermost electron from. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the. Which Type Of Electrons Are Best At Shielding A 3P Electron.
From spmchemistry.blog.onlinetuition.com.my
Electron Arrangement in Atom SPM Chemistry Which Type Of Electrons Are Best At Shielding A 3P Electron the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. the electron being removed from an \(\ce{al}\) atom is a \(3p\) electron, which is shielded by the two \(3s\) electrons, as well as all the inner. shielding is caused by the combination of partial. Which Type Of Electrons Are Best At Shielding A 3P Electron.