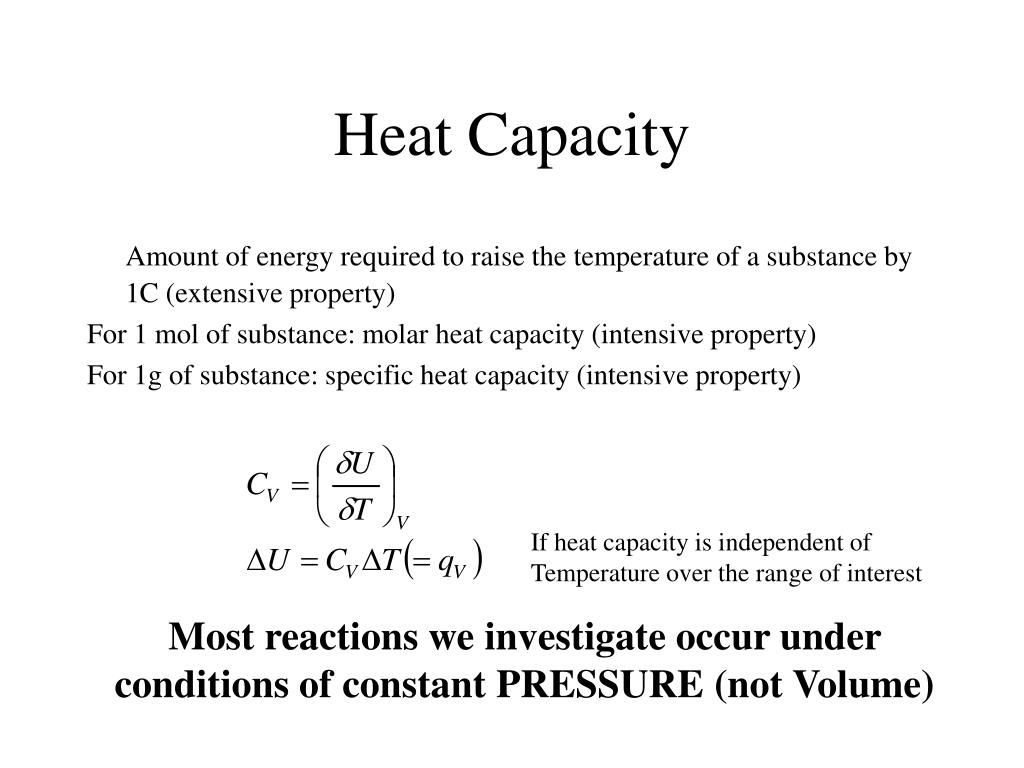
What Means Specific Heat Capacity . Specific heat, the quantity of heat required to raise the temperature of one gram of a substance by one celsius degree. It plays a crucial role in understanding. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. The specific heat capacity of a material is the energy required to raise one kilogram (kg) of the material by one degree celsius (°c). The units of specific heat are usually calories or joules per gram per celsius. The specific heat capacity is the amount of heat it takes to change the temperature of one gram of substance by 1°c. It is usually expressed as calories per degree in. Heat capacity, ratio of heat absorbed by a material to the temperature change.
from www.slideserve.com
Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. The specific heat capacity is the amount of heat it takes to change the temperature of one gram of substance by 1°c. It plays a crucial role in understanding. Specific heat, the quantity of heat required to raise the temperature of one gram of a substance by one celsius degree. The specific heat capacity of a material is the energy required to raise one kilogram (kg) of the material by one degree celsius (°c). Heat capacity, ratio of heat absorbed by a material to the temperature change. It is usually expressed as calories per degree in. The units of specific heat are usually calories or joules per gram per celsius.
PPT Heat Capacity PowerPoint Presentation, free download ID6674197
What Means Specific Heat Capacity Heat capacity, ratio of heat absorbed by a material to the temperature change. It plays a crucial role in understanding. Specific heat, the quantity of heat required to raise the temperature of one gram of a substance by one celsius degree. Heat capacity, ratio of heat absorbed by a material to the temperature change. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. It is usually expressed as calories per degree in. The specific heat capacity is the amount of heat it takes to change the temperature of one gram of substance by 1°c. The units of specific heat are usually calories or joules per gram per celsius. The specific heat capacity of a material is the energy required to raise one kilogram (kg) of the material by one degree celsius (°c).
From www.markedbyteachers.com
Specific Heat Capacity International Baccalaureate Physics Marked What Means Specific Heat Capacity The specific heat capacity is the amount of heat it takes to change the temperature of one gram of substance by 1°c. Heat capacity, ratio of heat absorbed by a material to the temperature change. It plays a crucial role in understanding. The units of specific heat are usually calories or joules per gram per celsius. Specific heat is defined. What Means Specific Heat Capacity.
From www.youtube.com
Chemistry 10.2 Specific Heat Capacity YouTube What Means Specific Heat Capacity The specific heat capacity is the amount of heat it takes to change the temperature of one gram of substance by 1°c. Heat capacity, ratio of heat absorbed by a material to the temperature change. It is usually expressed as calories per degree in. It plays a crucial role in understanding. Specific heat, the quantity of heat required to raise. What Means Specific Heat Capacity.
From study.com
Specific Heat Capacity Definition, Formula & Calculation Video What Means Specific Heat Capacity Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. Specific heat, the quantity of heat required to raise the temperature of one gram of a substance by one celsius degree. Heat capacity, ratio of heat absorbed by a material to the temperature change. The. What Means Specific Heat Capacity.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download What Means Specific Heat Capacity The units of specific heat are usually calories or joules per gram per celsius. The specific heat capacity is the amount of heat it takes to change the temperature of one gram of substance by 1°c. Specific heat, the quantity of heat required to raise the temperature of one gram of a substance by one celsius degree. Heat capacity, ratio. What Means Specific Heat Capacity.
From www.tes.com
Specific heat capacity complete lesson (GCSE 19) Teaching Resources What Means Specific Heat Capacity Heat capacity, ratio of heat absorbed by a material to the temperature change. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. The specific heat capacity is the amount of heat it takes to change the temperature of one gram of substance by 1°c.. What Means Specific Heat Capacity.
From www.toppr.com
Specific Heat Formula Definition, Equations, Examples What Means Specific Heat Capacity Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. It is usually expressed as calories per degree in. The specific heat capacity is the amount of heat it takes to change the temperature of one gram of substance by 1°c. It plays a crucial. What Means Specific Heat Capacity.
From www.youtube.com
What Is The Difference Between Specific Heat Capacity, Heat Capacity What Means Specific Heat Capacity The specific heat capacity of a material is the energy required to raise one kilogram (kg) of the material by one degree celsius (°c). It plays a crucial role in understanding. The specific heat capacity is the amount of heat it takes to change the temperature of one gram of substance by 1°c. Specific heat, the quantity of heat required. What Means Specific Heat Capacity.
From studylib.net
1.3 Specific Heat Capacity What Means Specific Heat Capacity Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. The specific heat capacity is the amount of heat it takes to change the temperature of one gram of substance by 1°c. Heat capacity, ratio of heat absorbed by a material to the temperature change.. What Means Specific Heat Capacity.
From www.slideserve.com
PPT Specific heat capacity PowerPoint Presentation, free download What Means Specific Heat Capacity The specific heat capacity is the amount of heat it takes to change the temperature of one gram of substance by 1°c. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. It is usually expressed as calories per degree in. Specific heat, the quantity. What Means Specific Heat Capacity.
From www.slideserve.com
PPT Specific Heat vs. Heat capacity PowerPoint Presentation, free What Means Specific Heat Capacity Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. Heat capacity, ratio of heat absorbed by a material to the temperature change. The specific heat capacity of a material is the energy required to raise one kilogram (kg) of the material by one degree. What Means Specific Heat Capacity.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download What Means Specific Heat Capacity The specific heat capacity of a material is the energy required to raise one kilogram (kg) of the material by one degree celsius (°c). Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. Heat capacity, ratio of heat absorbed by a material to the. What Means Specific Heat Capacity.
From www.youtube.com
Specific Heat and Heat Capacity YouTube What Means Specific Heat Capacity The specific heat capacity is the amount of heat it takes to change the temperature of one gram of substance by 1°c. It is usually expressed as calories per degree in. Specific heat, the quantity of heat required to raise the temperature of one gram of a substance by one celsius degree. Specific heat is defined as the amount of. What Means Specific Heat Capacity.
From www.markedbyteachers.com
Specific Heat Capacity International Baccalaureate Physics Marked What Means Specific Heat Capacity The units of specific heat are usually calories or joules per gram per celsius. The specific heat capacity of a material is the energy required to raise one kilogram (kg) of the material by one degree celsius (°c). The specific heat capacity is the amount of heat it takes to change the temperature of one gram of substance by 1°c.. What Means Specific Heat Capacity.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download What Means Specific Heat Capacity Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. Heat capacity, ratio of heat absorbed by a material to the temperature change. It is usually expressed as calories per degree in. It plays a crucial role in understanding. The specific heat capacity of a. What Means Specific Heat Capacity.
From abramsrewing.blogspot.com
Specific Heat Capacity Definition AbramsrEwing What Means Specific Heat Capacity Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. The specific heat capacity of a material is the energy required to raise one kilogram (kg) of the material by one degree celsius (°c). The specific heat capacity is the amount of heat it takes. What Means Specific Heat Capacity.
From www.youtube.com
Heat Capacity, Specific Heat Capacity Cambridge IGO level Physics 0625 What Means Specific Heat Capacity The specific heat capacity of a material is the energy required to raise one kilogram (kg) of the material by one degree celsius (°c). The specific heat capacity is the amount of heat it takes to change the temperature of one gram of substance by 1°c. Heat capacity, ratio of heat absorbed by a material to the temperature change. Specific. What Means Specific Heat Capacity.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download What Means Specific Heat Capacity Specific heat, the quantity of heat required to raise the temperature of one gram of a substance by one celsius degree. The units of specific heat are usually calories or joules per gram per celsius. It plays a crucial role in understanding. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass. What Means Specific Heat Capacity.
From scienceinfo.com
Specific Heat Capacity Definition, Unit, Formula What Means Specific Heat Capacity Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. Specific heat, the quantity of heat required to raise the temperature of one gram of a substance by one celsius degree. It is usually expressed as calories per degree in. The units of specific heat. What Means Specific Heat Capacity.
From www.geeksforgeeks.org
Specific Heat Capacity Definition, Formula, Water Heat Capacity What Means Specific Heat Capacity It plays a crucial role in understanding. Heat capacity, ratio of heat absorbed by a material to the temperature change. The units of specific heat are usually calories or joules per gram per celsius. The specific heat capacity is the amount of heat it takes to change the temperature of one gram of substance by 1°c. Specific heat, the quantity. What Means Specific Heat Capacity.
From studymind.co.uk
Heat Capacity Study Mind What Means Specific Heat Capacity Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. Specific heat, the quantity of heat required to raise the temperature of one gram of a substance by one celsius degree. It plays a crucial role in understanding. The specific heat capacity of a material. What Means Specific Heat Capacity.
From www.slideserve.com
PPT Heat capacity and specific heat capacity PowerPoint Presentation What Means Specific Heat Capacity The specific heat capacity of a material is the energy required to raise one kilogram (kg) of the material by one degree celsius (°c). Specific heat, the quantity of heat required to raise the temperature of one gram of a substance by one celsius degree. The specific heat capacity is the amount of heat it takes to change the temperature. What Means Specific Heat Capacity.
From www.slideserve.com
PPT Specific heat capacity PowerPoint Presentation, free download What Means Specific Heat Capacity The specific heat capacity is the amount of heat it takes to change the temperature of one gram of substance by 1°c. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. Heat capacity, ratio of heat absorbed by a material to the temperature change.. What Means Specific Heat Capacity.
From www.britannica.com
Specific heat physics Britannica What Means Specific Heat Capacity The specific heat capacity of a material is the energy required to raise one kilogram (kg) of the material by one degree celsius (°c). It plays a crucial role in understanding. The units of specific heat are usually calories or joules per gram per celsius. Specific heat is defined as the amount of heat required to raise the temperature of. What Means Specific Heat Capacity.
From www.slideserve.com
PPT Heat Capacity PowerPoint Presentation, free download ID6674197 What Means Specific Heat Capacity It is usually expressed as calories per degree in. Specific heat, the quantity of heat required to raise the temperature of one gram of a substance by one celsius degree. The specific heat capacity of a material is the energy required to raise one kilogram (kg) of the material by one degree celsius (°c). Specific heat is defined as the. What Means Specific Heat Capacity.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download What Means Specific Heat Capacity Specific heat, the quantity of heat required to raise the temperature of one gram of a substance by one celsius degree. The specific heat capacity of a material is the energy required to raise one kilogram (kg) of the material by one degree celsius (°c). Heat capacity, ratio of heat absorbed by a material to the temperature change. Specific heat. What Means Specific Heat Capacity.
From www.sciencefacts.net
Specific Heat and Heat Capacity Definition, Formula, Values, and Problems What Means Specific Heat Capacity It plays a crucial role in understanding. The specific heat capacity of a material is the energy required to raise one kilogram (kg) of the material by one degree celsius (°c). Heat capacity, ratio of heat absorbed by a material to the temperature change. The specific heat capacity is the amount of heat it takes to change the temperature of. What Means Specific Heat Capacity.
From worksheetlibrarysteins.z19.web.core.windows.net
Formula To Calculate Specific Heat What Means Specific Heat Capacity Specific heat, the quantity of heat required to raise the temperature of one gram of a substance by one celsius degree. It is usually expressed as calories per degree in. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. The specific heat capacity of. What Means Specific Heat Capacity.
From www.slideshare.net
Heat Capacity What Means Specific Heat Capacity The units of specific heat are usually calories or joules per gram per celsius. Heat capacity, ratio of heat absorbed by a material to the temperature change. It plays a crucial role in understanding. The specific heat capacity is the amount of heat it takes to change the temperature of one gram of substance by 1°c. The specific heat capacity. What Means Specific Heat Capacity.
From testbook.com
Specific Heat Capacity Learning Notes for IIT JEE Testbook What Means Specific Heat Capacity The units of specific heat are usually calories or joules per gram per celsius. Heat capacity, ratio of heat absorbed by a material to the temperature change. Specific heat, the quantity of heat required to raise the temperature of one gram of a substance by one celsius degree. The specific heat capacity is the amount of heat it takes to. What Means Specific Heat Capacity.
From www.slideserve.com
PPT Heat Capacity and Specific Heat Capacity PowerPoint Presentation What Means Specific Heat Capacity It plays a crucial role in understanding. Specific heat, the quantity of heat required to raise the temperature of one gram of a substance by one celsius degree. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. The specific heat capacity of a material. What Means Specific Heat Capacity.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download What Means Specific Heat Capacity The specific heat capacity is the amount of heat it takes to change the temperature of one gram of substance by 1°c. The specific heat capacity of a material is the energy required to raise one kilogram (kg) of the material by one degree celsius (°c). The units of specific heat are usually calories or joules per gram per celsius.. What Means Specific Heat Capacity.
From askfilo.com
The differences between specific heat capacity and heat capacity are.. What Means Specific Heat Capacity The units of specific heat are usually calories or joules per gram per celsius. It plays a crucial role in understanding. It is usually expressed as calories per degree in. The specific heat capacity of a material is the energy required to raise one kilogram (kg) of the material by one degree celsius (°c). Specific heat is defined as the. What Means Specific Heat Capacity.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download What Means Specific Heat Capacity Specific heat, the quantity of heat required to raise the temperature of one gram of a substance by one celsius degree. Heat capacity, ratio of heat absorbed by a material to the temperature change. The specific heat capacity is the amount of heat it takes to change the temperature of one gram of substance by 1°c. The specific heat capacity. What Means Specific Heat Capacity.
From www.youtube.com
Specific heat capacity YouTube What Means Specific Heat Capacity The specific heat capacity of a material is the energy required to raise one kilogram (kg) of the material by one degree celsius (°c). Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. The units of specific heat are usually calories or joules per. What Means Specific Heat Capacity.
From slidetodoc.com
Specific Heat Capacity The specific heat capacity of What Means Specific Heat Capacity Specific heat, the quantity of heat required to raise the temperature of one gram of a substance by one celsius degree. It plays a crucial role in understanding. The units of specific heat are usually calories or joules per gram per celsius. The specific heat capacity is the amount of heat it takes to change the temperature of one gram. What Means Specific Heat Capacity.