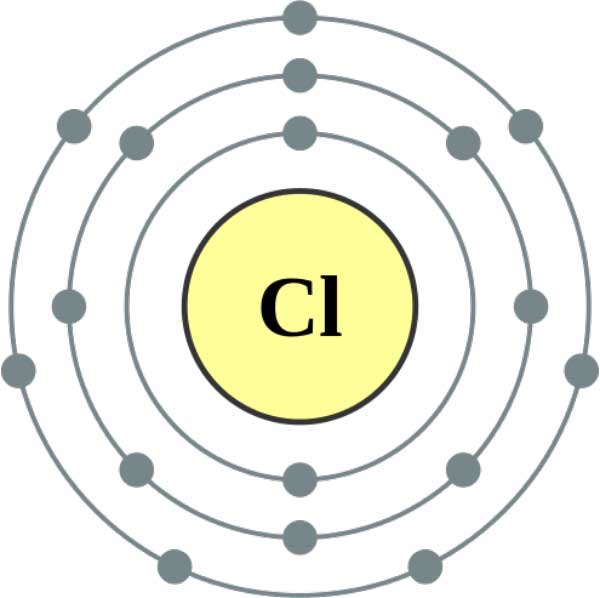
Chlorine Chloride Ion Radius . There are several other ways ways to define radius for atoms and ions. The atomic radius of the chlorine atom (cl) is 79 pm, and the ionic radius of chloride. The atomic radius of the sodium atom (na) is 190 pm, and the ionic radius of sodium ion (na +) is 116 pm. Ions may be larger or smaller than the neutral atom, depending on the ion's charge. Ionic radii are difficult to measure with any degree of certainty, and vary according to the environment of the ion. An atom such as chlorine has both a covalent radius (the distance between the two atoms in a \(\ce{cl2}\) molecule) and a van der waals radius (the. Typical values range from 30åpm (0.3åä) to over 200åpm (2åä). This page explains the various measures of atomic radius, and then looks at the way it varies around the periodic. Due to the difference in the. Conclusion, chloride ion has a bigger atomic radius due to an increase in number of electrons. The bond length in clcl is:
from loenqakuj.blob.core.windows.net
Ions may be larger or smaller than the neutral atom, depending on the ion's charge. There are several other ways ways to define radius for atoms and ions. An atom such as chlorine has both a covalent radius (the distance between the two atoms in a \(\ce{cl2}\) molecule) and a van der waals radius (the. Typical values range from 30åpm (0.3åä) to over 200åpm (2åä). The atomic radius of the sodium atom (na) is 190 pm, and the ionic radius of sodium ion (na +) is 116 pm. Ionic radii are difficult to measure with any degree of certainty, and vary according to the environment of the ion. The atomic radius of the chlorine atom (cl) is 79 pm, and the ionic radius of chloride. Due to the difference in the. This page explains the various measures of atomic radius, and then looks at the way it varies around the periodic. Conclusion, chloride ion has a bigger atomic radius due to an increase in number of electrons.
Chlorine Number For Atomic Mass at Pamela Lansing blog
Chlorine Chloride Ion Radius Typical values range from 30åpm (0.3åä) to over 200åpm (2åä). The atomic radius of the sodium atom (na) is 190 pm, and the ionic radius of sodium ion (na +) is 116 pm. The bond length in clcl is: This page explains the various measures of atomic radius, and then looks at the way it varies around the periodic. There are several other ways ways to define radius for atoms and ions. Conclusion, chloride ion has a bigger atomic radius due to an increase in number of electrons. Typical values range from 30åpm (0.3åä) to over 200åpm (2åä). An atom such as chlorine has both a covalent radius (the distance between the two atoms in a \(\ce{cl2}\) molecule) and a van der waals radius (the. Due to the difference in the. Ionic radii are difficult to measure with any degree of certainty, and vary according to the environment of the ion. Ions may be larger or smaller than the neutral atom, depending on the ion's charge. The atomic radius of the chlorine atom (cl) is 79 pm, and the ionic radius of chloride.
From sciencenotes.org
Atomic Radius and Ionic Radius Chlorine Chloride Ion Radius Conclusion, chloride ion has a bigger atomic radius due to an increase in number of electrons. An atom such as chlorine has both a covalent radius (the distance between the two atoms in a \(\ce{cl2}\) molecule) and a van der waals radius (the. This page explains the various measures of atomic radius, and then looks at the way it varies. Chlorine Chloride Ion Radius.
From www.doubtnut.com
(a) Give the schematic atomic structures of chlorine atom and chloride Chlorine Chloride Ion Radius Conclusion, chloride ion has a bigger atomic radius due to an increase in number of electrons. An atom such as chlorine has both a covalent radius (the distance between the two atoms in a \(\ce{cl2}\) molecule) and a van der waals radius (the. Typical values range from 30åpm (0.3åä) to over 200åpm (2åä). Ions may be larger or smaller than. Chlorine Chloride Ion Radius.
From www.slideserve.com
PPT Mason University General Chemistry 211 Chapter 9 Models of Chemical Bonding Chlorine Chloride Ion Radius There are several other ways ways to define radius for atoms and ions. An atom such as chlorine has both a covalent radius (the distance between the two atoms in a \(\ce{cl2}\) molecule) and a van der waals radius (the. Ions may be larger or smaller than the neutral atom, depending on the ion's charge. Due to the difference in. Chlorine Chloride Ion Radius.
From stock.adobe.com
Diagram explaining Atomic Radius using diatomic molecules. Oxygen, hydrogen, nitrogen, fluorine Chlorine Chloride Ion Radius The atomic radius of the chlorine atom (cl) is 79 pm, and the ionic radius of chloride. Due to the difference in the. There are several other ways ways to define radius for atoms and ions. Ionic radii are difficult to measure with any degree of certainty, and vary according to the environment of the ion. This page explains the. Chlorine Chloride Ion Radius.
From newtondesk.com
Chlorine Cl (Element 17) of Periodic Table Chlorine Chloride Ion Radius Due to the difference in the. Typical values range from 30åpm (0.3åä) to over 200åpm (2åä). The bond length in clcl is: Ionic radii are difficult to measure with any degree of certainty, and vary according to the environment of the ion. Ions may be larger or smaller than the neutral atom, depending on the ion's charge. The atomic radius. Chlorine Chloride Ion Radius.
From www.slideserve.com
PPT Unit 4 The Periodic Table and Periodicity PowerPoint Presentation ID1588480 Chlorine Chloride Ion Radius This page explains the various measures of atomic radius, and then looks at the way it varies around the periodic. Typical values range from 30åpm (0.3åä) to over 200åpm (2åä). The atomic radius of the sodium atom (na) is 190 pm, and the ionic radius of sodium ion (na +) is 116 pm. Due to the difference in the. Ions. Chlorine Chloride Ion Radius.
From sciencenotes.org
Chlorine Facts Chlorine Chloride Ion Radius There are several other ways ways to define radius for atoms and ions. Typical values range from 30åpm (0.3åä) to over 200åpm (2åä). The atomic radius of the chlorine atom (cl) is 79 pm, and the ionic radius of chloride. An atom such as chlorine has both a covalent radius (the distance between the two atoms in a \(\ce{cl2}\) molecule). Chlorine Chloride Ion Radius.
From www.bartleby.com
Answered Table 12.3 Ionic Radii for Several… bartleby Chlorine Chloride Ion Radius Conclusion, chloride ion has a bigger atomic radius due to an increase in number of electrons. Due to the difference in the. Ions may be larger or smaller than the neutral atom, depending on the ion's charge. The atomic radius of the sodium atom (na) is 190 pm, and the ionic radius of sodium ion (na +) is 116 pm.. Chlorine Chloride Ion Radius.
From www.britannica.com
ionic bond Definition, Properties, Examples, & Facts Britannica Chlorine Chloride Ion Radius The atomic radius of the sodium atom (na) is 190 pm, and the ionic radius of sodium ion (na +) is 116 pm. There are several other ways ways to define radius for atoms and ions. Conclusion, chloride ion has a bigger atomic radius due to an increase in number of electrons. Typical values range from 30åpm (0.3åä) to over. Chlorine Chloride Ion Radius.
From www.crystalmaker.com
Elements, Atomic Radii and the Periodic Radii Chlorine Chloride Ion Radius Typical values range from 30åpm (0.3åä) to over 200åpm (2åä). The bond length in clcl is: The atomic radius of the chlorine atom (cl) is 79 pm, and the ionic radius of chloride. This page explains the various measures of atomic radius, and then looks at the way it varies around the periodic. Ions may be larger or smaller than. Chlorine Chloride Ion Radius.
From material-properties.org
Chlorine Periodic Table and Atomic Properties Chlorine Chloride Ion Radius Ions may be larger or smaller than the neutral atom, depending on the ion's charge. Typical values range from 30åpm (0.3åä) to over 200åpm (2åä). The atomic radius of the sodium atom (na) is 190 pm, and the ionic radius of sodium ion (na +) is 116 pm. This page explains the various measures of atomic radius, and then looks. Chlorine Chloride Ion Radius.
From scientifictutor.org
Chem College Ionic Radius (Ionic Size) Scientific Tutor Chlorine Chloride Ion Radius The atomic radius of the sodium atom (na) is 190 pm, and the ionic radius of sodium ion (na +) is 116 pm. The atomic radius of the chlorine atom (cl) is 79 pm, and the ionic radius of chloride. This page explains the various measures of atomic radius, and then looks at the way it varies around the periodic.. Chlorine Chloride Ion Radius.
From www.embibe.com
Draw the atomic structure of the Chlorine atom and chlorine ion Chlorine Chloride Ion Radius Ions may be larger or smaller than the neutral atom, depending on the ion's charge. An atom such as chlorine has both a covalent radius (the distance between the two atoms in a \(\ce{cl2}\) molecule) and a van der waals radius (the. There are several other ways ways to define radius for atoms and ions. Typical values range from 30åpm. Chlorine Chloride Ion Radius.
From www.alamy.com
Chlorine electron configuration. Illustration of the atomic structure and electron configuration Chlorine Chloride Ion Radius Ions may be larger or smaller than the neutral atom, depending on the ion's charge. Due to the difference in the. There are several other ways ways to define radius for atoms and ions. This page explains the various measures of atomic radius, and then looks at the way it varies around the periodic. The atomic radius of the chlorine. Chlorine Chloride Ion Radius.
From differencebtw.com
Chlorine Atom vs. Chloride Ion Know the Difference Chlorine Chloride Ion Radius Typical values range from 30åpm (0.3åä) to over 200åpm (2åä). Ions may be larger or smaller than the neutral atom, depending on the ion's charge. This page explains the various measures of atomic radius, and then looks at the way it varies around the periodic. Ionic radii are difficult to measure with any degree of certainty, and vary according to. Chlorine Chloride Ion Radius.
From pediaa.com
Difference Between Chlorine and Chloride Definition, Properties, Reactions, Applications Chlorine Chloride Ion Radius Due to the difference in the. The atomic radius of the chlorine atom (cl) is 79 pm, and the ionic radius of chloride. The bond length in clcl is: This page explains the various measures of atomic radius, and then looks at the way it varies around the periodic. There are several other ways ways to define radius for atoms. Chlorine Chloride Ion Radius.
From periodictable.me
Chlorine Electron Configuration (Cl) with Orbital Diagram Chlorine Chloride Ion Radius An atom such as chlorine has both a covalent radius (the distance between the two atoms in a \(\ce{cl2}\) molecule) and a van der waals radius (the. This page explains the various measures of atomic radius, and then looks at the way it varies around the periodic. Typical values range from 30åpm (0.3åä) to over 200åpm (2åä). Due to the. Chlorine Chloride Ion Radius.
From www.slideserve.com
PPT Seawater Chemistry PowerPoint Presentation, free download ID1073512 Chlorine Chloride Ion Radius The bond length in clcl is: An atom such as chlorine has both a covalent radius (the distance between the two atoms in a \(\ce{cl2}\) molecule) and a van der waals radius (the. Due to the difference in the. The atomic radius of the sodium atom (na) is 190 pm, and the ionic radius of sodium ion (na +) is. Chlorine Chloride Ion Radius.
From www.shutterstock.com
585 Chlorine electrons 이미지, 스톡 사진 및 벡터 Shutterstock Chlorine Chloride Ion Radius The bond length in clcl is: Conclusion, chloride ion has a bigger atomic radius due to an increase in number of electrons. There are several other ways ways to define radius for atoms and ions. Ionic radii are difficult to measure with any degree of certainty, and vary according to the environment of the ion. Typical values range from 30åpm. Chlorine Chloride Ion Radius.
From www.bartleby.com
B. A chlorine atom (Cl) a negatively charged chloride ion (Cl − ) when it gains an Chlorine Chloride Ion Radius This page explains the various measures of atomic radius, and then looks at the way it varies around the periodic. There are several other ways ways to define radius for atoms and ions. Due to the difference in the. The bond length in clcl is: The atomic radius of the sodium atom (na) is 190 pm, and the ionic radius. Chlorine Chloride Ion Radius.
From www.dreamstime.com
An Atom of Chlorine Diagram Stock Vector Illustration of structure, chemistry 141346692 Chlorine Chloride Ion Radius An atom such as chlorine has both a covalent radius (the distance between the two atoms in a \(\ce{cl2}\) molecule) and a van der waals radius (the. This page explains the various measures of atomic radius, and then looks at the way it varies around the periodic. There are several other ways ways to define radius for atoms and ions.. Chlorine Chloride Ion Radius.
From inspiritvr.com
Ionic radii Study Guide Inspirit Chlorine Chloride Ion Radius The atomic radius of the sodium atom (na) is 190 pm, and the ionic radius of sodium ion (na +) is 116 pm. The atomic radius of the chlorine atom (cl) is 79 pm, and the ionic radius of chloride. There are several other ways ways to define radius for atoms and ions. The bond length in clcl is: Typical. Chlorine Chloride Ion Radius.
From www.animalia-life.club
Chloride Ion Number Of Protons And Electrons Chlorine Chloride Ion Radius Due to the difference in the. Ionic radii are difficult to measure with any degree of certainty, and vary according to the environment of the ion. Typical values range from 30åpm (0.3åä) to over 200åpm (2åä). Ions may be larger or smaller than the neutral atom, depending on the ion's charge. Conclusion, chloride ion has a bigger atomic radius due. Chlorine Chloride Ion Radius.
From www.dreamstime.com
Diagram Representation Of The Element Chlorine Stock Illustration Image 59013880 Chlorine Chloride Ion Radius Ions may be larger or smaller than the neutral atom, depending on the ion's charge. The atomic radius of the sodium atom (na) is 190 pm, and the ionic radius of sodium ion (na +) is 116 pm. Typical values range from 30åpm (0.3åä) to over 200åpm (2åä). The atomic radius of the chlorine atom (cl) is 79 pm, and. Chlorine Chloride Ion Radius.
From courses.lumenlearning.com
Periodic Variations in Element Properties CHEM 1305 General Chemistry I—Lecture Chlorine Chloride Ion Radius There are several other ways ways to define radius for atoms and ions. The atomic radius of the chlorine atom (cl) is 79 pm, and the ionic radius of chloride. This page explains the various measures of atomic radius, and then looks at the way it varies around the periodic. Conclusion, chloride ion has a bigger atomic radius due to. Chlorine Chloride Ion Radius.
From www.britannica.com
Chlorine Uses, Properties, & Facts Britannica Chlorine Chloride Ion Radius Due to the difference in the. There are several other ways ways to define radius for atoms and ions. Conclusion, chloride ion has a bigger atomic radius due to an increase in number of electrons. Ions may be larger or smaller than the neutral atom, depending on the ion's charge. This page explains the various measures of atomic radius, and. Chlorine Chloride Ion Radius.
From gia-kharmon.blogspot.com
What Is the Atomic Radius of Chlorine Chlorine Chloride Ion Radius An atom such as chlorine has both a covalent radius (the distance between the two atoms in a \(\ce{cl2}\) molecule) and a van der waals radius (the. The atomic radius of the sodium atom (na) is 190 pm, and the ionic radius of sodium ion (na +) is 116 pm. Conclusion, chloride ion has a bigger atomic radius due to. Chlorine Chloride Ion Radius.
From ecurrencythailand.com
When A Chlorine Atom Forms An Ion Its Radius? 10 Most Correct Answers Chlorine Chloride Ion Radius The atomic radius of the chlorine atom (cl) is 79 pm, and the ionic radius of chloride. The atomic radius of the sodium atom (na) is 190 pm, and the ionic radius of sodium ion (na +) is 116 pm. An atom such as chlorine has both a covalent radius (the distance between the two atoms in a \(\ce{cl2}\) molecule). Chlorine Chloride Ion Radius.
From www.chegg.com
Solved Given that the ionic radii of Sodium, Na+, and Chlorine Chloride Ion Radius Conclusion, chloride ion has a bigger atomic radius due to an increase in number of electrons. Due to the difference in the. Ions may be larger or smaller than the neutral atom, depending on the ion's charge. The atomic radius of the chlorine atom (cl) is 79 pm, and the ionic radius of chloride. This page explains the various measures. Chlorine Chloride Ion Radius.
From chemistry.stackexchange.com
What is the atomic radius of chlorine? Chemistry Stack Exchange Chlorine Chloride Ion Radius Conclusion, chloride ion has a bigger atomic radius due to an increase in number of electrons. The bond length in clcl is: Ionic radii are difficult to measure with any degree of certainty, and vary according to the environment of the ion. Ions may be larger or smaller than the neutral atom, depending on the ion's charge. An atom such. Chlorine Chloride Ion Radius.
From dxorxejky.blob.core.windows.net
Does Chlorine Have A Larger Atomic Radius Than Fluorine at Leon Cain blog Chlorine Chloride Ion Radius An atom such as chlorine has both a covalent radius (the distance between the two atoms in a \(\ce{cl2}\) molecule) and a van der waals radius (the. Typical values range from 30åpm (0.3åä) to over 200åpm (2åä). The atomic radius of the chlorine atom (cl) is 79 pm, and the ionic radius of chloride. Due to the difference in the.. Chlorine Chloride Ion Radius.
From www.scienceforums.net
Chemistry Comparing Ionic Radii of Cs+ and Cl ions Homework Help Science Forums Chlorine Chloride Ion Radius Ions may be larger or smaller than the neutral atom, depending on the ion's charge. The atomic radius of the sodium atom (na) is 190 pm, and the ionic radius of sodium ion (na +) is 116 pm. This page explains the various measures of atomic radius, and then looks at the way it varies around the periodic. The bond. Chlorine Chloride Ion Radius.
From www.youtube.com
Cl(Chloride ion) and Cl(Chlorine) Electron Configuration,Orbital Diagrams,Shorthand/Longhand Chlorine Chloride Ion Radius The bond length in clcl is: An atom such as chlorine has both a covalent radius (the distance between the two atoms in a \(\ce{cl2}\) molecule) and a van der waals radius (the. The atomic radius of the chlorine atom (cl) is 79 pm, and the ionic radius of chloride. Typical values range from 30åpm (0.3åä) to over 200åpm (2åä).. Chlorine Chloride Ion Radius.
From ar.inspiredpencil.com
Periodic Table Ionic Radius Chlorine Chloride Ion Radius Due to the difference in the. There are several other ways ways to define radius for atoms and ions. The atomic radius of the chlorine atom (cl) is 79 pm, and the ionic radius of chloride. Ions may be larger or smaller than the neutral atom, depending on the ion's charge. Typical values range from 30åpm (0.3åä) to over 200åpm. Chlorine Chloride Ion Radius.
From loenqakuj.blob.core.windows.net
Chlorine Number For Atomic Mass at Pamela Lansing blog Chlorine Chloride Ion Radius This page explains the various measures of atomic radius, and then looks at the way it varies around the periodic. Due to the difference in the. Ionic radii are difficult to measure with any degree of certainty, and vary according to the environment of the ion. An atom such as chlorine has both a covalent radius (the distance between the. Chlorine Chloride Ion Radius.